Carbapenems and aztreonam
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Antibiotics And Synthetic Antimicrobial Agents: Their Properties And Uses
The spread of organisms that had developed various forms of resistance to the early penicillins and cephalosporins lead to a search for other β-lactam antibiotics that avoided these resistance problems.
Carbapenems and aztreonam
The spread of organisms that had
developed various forms of resistance to the early penicillins and
cephalosporins lead to a search for other β-lactam antibiotics that avoided
these resistance problems. Many naturally occurring and synthetic compounds
were examined but only a few were developed to become marketed products, and
the most useful of these were the carbapenems. This group may be considered as
penicillin or cephalosporin derivatives in which the sulphur atom has been
replaced with a carbon. The nomenclature is confusing because some reference sources
used terms like carbapenems, olivanic acids and thienamycins as if they were
synonymous. In fact, carbapenems is the generic term for the group which
includes olivanic acids (of which there are no products in therapeutic use) and
the thienamycins. The earliest thienamycins were discovered in the 1970s but
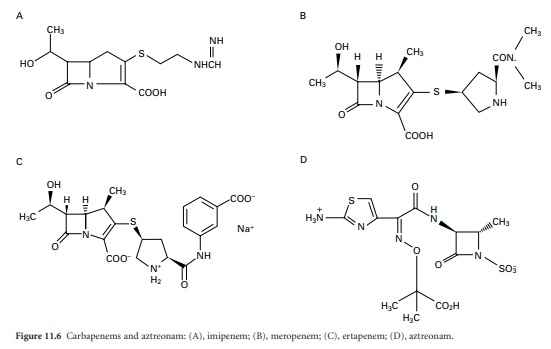
proved difficult to develop because of their poor stability. The N-formimidoyl derivative of thienamycin, which was
named imipenem (Figure 11.6A),
proved to combine the desirable properties of in vitro stability,
a broad spectrum of antimicrobial activity and good resistance to almost all of
the then known β-lactamases. Its only shortcoming was poor in vivo-stability because it was vulnerable to
hydrolysis by mammalian renal dipeptidase, but this was solved by the
development of a renal dipeptidase inhibitor, cilastatin, with which imipenem
was marketed. Meropenem, marketed more recently, is more stable than imipenem
to dipeptidase and may thus be administered without cilastatin; its chemical
structure is depicted in Figure 11.6B.
Ertapenem (Figure 11.6C)
has properties similar to those of meropenem but affords the additional
advantage of once daily dosing.
Examination of the structure-activity
relationships of the early β-lactam antibiotics led to an expectation that
molecules possessing only the β-lactam ring without a second ring fused to it
would have no antimicrobial activity. This proved not to be so when such
naturally occurring antibiotics, termed monobactams, were discovered, and found
not only to possess activity, but to exhibit good resistance to β-lactamases.
The naturally occurring monobactams were not developed for clinical use, but an
analogue, aztreonam, produced totally by conventional chemical synthesis, was
marketed in 1986. It is highly active against most Gram-negative bacteria and
stable to most types of β-lactamases, although its resistance to staphylococcal
β-lactamases is irrelevant because it is inactive against all strains of Staph. aureus as well as other Gram-positive
species and anaerobes. Like the carbapenems, it is formulated as an intravenous
injection and, as such, it tends to be limited to use in hospitals for the
treatment of serious Gram-negative infections, including those due to Ps. aeruginosa. As with the carbapenems and
third generation cephalosporins, it exhibits synergy with aminoglycosides like
gentamicin and tobramycin, and these combinations are employed for the
treatment of Pseudomonas lung infections in
cystic fibrosis.
