Macrolides
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Antibiotics And Synthetic Antimicrobial Agents: Their Properties And Uses
The macrolide antibiotics are large molecules comprising 12–16-membered lactone rings linked through glycosidic bonds with amino sugars. Erythromycin was the first member of the group to be discovered in 1952 and it is still an important antibiotic today.
MACROLIDES
The macrolide antibiotics are large
molecules comprising 12–16-membered lactone rings
linked through glycosidic bonds with amino sugars. Erythromycin was the first member of the group to be discovered in 1952 and it is still an important
antibiotic today.
It was quickly followed 2 years later by spiramycin and oleandomycin but, although still available
in certain countries, these last two are now little used. Erythromycin suffers from several disadvantages: its antimicrobial
spectrum is largely restricted to Gram-positive species, it has
poor acid stability so its absorption is erratic, it commonly exhibits gastrointestinal side effects and bacteria acquire resistance to it relatively easily. These
shortcomings prompted the search
for new macrolides, and several semisynthetic derivatives were forthcoming: roxithromycin was marketed in 1987, clarithromycin and azithromycin in 1991 and the most recent,
telithromycin, in 2001.
Erythromycin (Figure 11.8) and roxithromycin are chemically similar
in possessing a 14-membered ring structure. A distinction is sometimes drawn
between them and both azithromycin which, strictly speaking, is an azalide (a 15-membered
ring containing an additional nitrogen
atom) and telithromycin which is a ketolide (a 14-membered ring
with an additional keto
group). The term
macrolide, however, is commonly used to describe
all five antibiotics, and that terminology will be used here.
The macrolides are
active against most Gram-positive
bacteria, Neisseria and H. influenzae but, with the excep tion of azithromycin, not against the Enterobacteriaceae.
Because their antibacterial spectrum is similar to those of the early penicillins, the macrolides were,
and still are, considered
alternatives for patients
with penicillin allergy. They are commonly
used for respiratory, skin and soft tissue infections, but one of the factors that stimulated
the development of the more
recent macrolides was
their activity
against emerging pathogens like species of Legionella, Campylobacter,
Helicobacter and Chlamydia, as well as some mycoplasmas and rickettsias and the
Mycobacterium avium complex to which AIDS/HIV patients are susceptible. The semisynthetic macrolides do not afford
a significant advantage over erythromycin in terms of their activity against
staphylococci, streptococci and enterococci, but they represent an
advantage in several other respects: they are generally more active against the other organisms mentioned
above; they exhibit better stability and pharmacokinetics,
thus permitting less frequent dosage
and better tissue
penetration; they
generally have fewer side effects;
and, particularly in the case of telithromycin and other ketolides,
they may be active against
some strains that have acquired resistance to erythromycin, and
they are, themselves, less vulnerable to resistance development.
The macrolides all act by inhibiting protein
synthesis in bacteria and
they are regarded as bacteristatic drugs, although bactericidal activity may be achieved
at high concentrations. The antimicrobial activity of
erythromycin is pH-dependent, increasing with pH up to about
8.5, and the same
effect occurs to varying degrees
with other members of the group.
The macrolides are extremely bitter and their
tablets are often
coated, both to disguise
the taste and to protect
the antibiotic from stomach acid. Erythromycin exhibits particularly
poor acid stability and erratic oral absorption, and a variety
of esters have been used to minimize these problems which,
although present, are much less evident in the semisynthetic molecules.
All the macrolides are orally active
and they are concentrated intracellularly, particularly into neutrophils by which they are
transported to infection sites. The longer elimination half-lives of the newer
drugs permit less
frequent dosing than that required
for erythromycin. The group as a whole are regarded as relatively safe
antibiotics which do not exhibit
severe adverse reactions, although gastrointestinal disturbances (nausea,
vomiting, abdominal pain and, infrequently, diarrhoea) are relatively common with erythromycin and much reduced
or absent in the others.
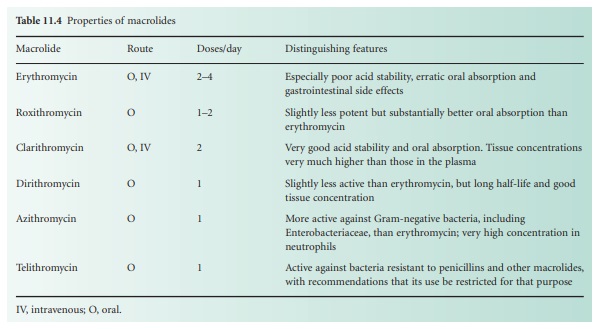
These and other
characteristics are summarized in Table 11.4.
Related Topics
