Sulphonamides, Trimethoprim and Related Drugs
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Antibiotics And Synthetic Antimicrobial Agents: Their Properties And Uses
Sulphonamides were discovered by Domagk in 1935. It had been shown that a red azo dye, prontosil, had a curative effect on mice infected with β-haemolytic streptococci; it was subsequently found that in vivo, prontosil was converted into sulphanilamide.
SULPHONAMIDES, TRIMETHOPRIM AND RELATED
DRUGS
Sulphonamides were
discovered by Domagk
in 1935. It had
been shown that
a red azo dye, prontosil, had a curative effect on mice infected with β-haemolytic streptococci; it was subsequently found that in vivo,
prontosil was converted into
sulphanilamide (Figure 11.9A).
The basis of the antimicrobial activity
of the sulphonamides is their
structural similarity to p-aminobenzoic acid (PABA) which
is an integral part of the B vitamin, folic acid. In sensitive bacteria, sulphonamides compete with PABA with the result
that folic acid
synthesis is reduced. Because the vitamin is essential for the manufacture of nucleic acids
(and other important biochemicals), this leads to a reduction in, or cessation
of, bacterial growth.
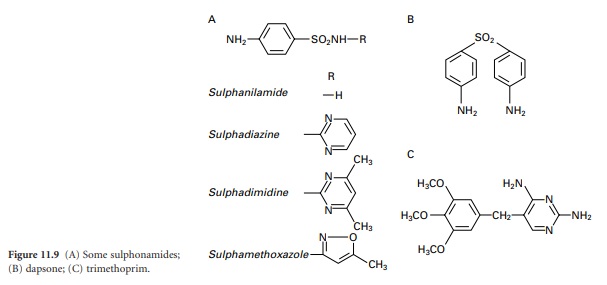
Chemical modifications
of sulphanilamide (see Figure 11.9A) gave compounds
with higher antibacterial activity or special properties like prolonged activity. The drugs were extensively used from the 1930s to the 1970s,
after which their popularity declined due to resistance development and the introduction of safer and more effective antibiotics.
A few sulphonamides are still used in topical
therapies(notably silver sulphadiazine for burns) and in veterinary medicine,
while sulphadiazine itself remains available for the
prevention of rheumatic fever. Dapsone (Figure 11.9B) is a sulphonamide derivative that has been
used extensively in the past for the treatment of leprosy, but again, although
still available and currently employed as part of a multidrug treatment for leprosy, its use has declined as a result
of resistance development and the introduction of better antibiotics.
In the
body, folic acid must
be reduced to dihydrofolic
acid and then tetrahydrofolic acid in order to
become active, and it was discovered that a group of synthetic drugs called diaminopyrimidines could inhibit the enzymes responsible for this reduction. These dihydrofolate reductase inhibitors, of which trimethoprim
(11.9C) is the most important, were found to act synergistically with sulphonamides because they blocked
successive steps in the synthesis of reduced folic
acid. Consequently trimethoprim was introduced in 1969 as a
combination product
with sulphamethoxazole (cotrimoxazole) for the treatment of urinary tract
and, less commonly, respiratory infections.
Unfortunately, the advantages of using the combination product
were not as great as anticipated partly because
pharmacokinetic differences between the
two drugs resulted
in relative concentrations in the body
which were far from optimal
for synergy. This, together with increasing evidence
that the antibacterial activity
of co-trimoxazole was due largely
to the trimethoprim component with the more toxic sulphamethoxazole
contributing little, lead to the use of trimethoprim alone from the mid 1970s.
Co-trimoxazole is currently recommended only for treatment of pneumocystis pneumonia, toxoplasmosis and nocardiasis.
Trimethoprim remains one of the least expensive
orally active
agents available for the treatment of urinary tract infections, for which it is still widely prescribed, although it, too, is suffering from
increasing resistance
development and the trend is towards its replacement
with fluoroquinolones (see section 6). Other trimethoprim analogues, notably tetroxoprim, have been introduced as antibacterial agents, but have not demonstrated
significant advantages
over trimethoprim itself. The other important diaminopyrimidine possessing antimicrobial activity is pyrimethamine. This is used
in combination with sulphadoxine or other drugs
for the treatment (but not any longer for the prophylaxis) of malaria. It is
used on its own for
the treatment of toxoplasmosis and pneumocystis
pneumonia.
Related Topics
