Penicillins
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Antibiotics And Synthetic Antimicrobial Agents: Their Properties And Uses
The older penicillins, benzylpenicillin and phenoxymethylpenicillin, are primarily active against Gram-positive bacteria; they are also β-lactamase sensitive.
PENICILLINS
The penicillins (general structure
shown in Figure 11.1A)
may be considered as being of the following types:
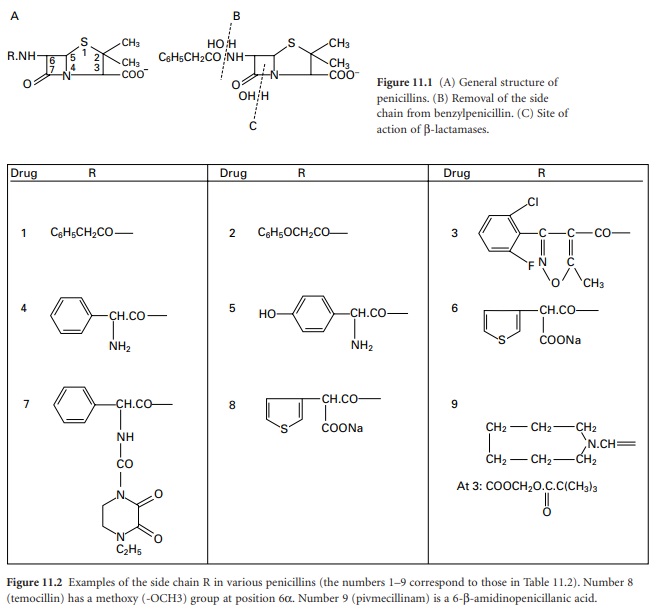
Naturally occurring.
For example, those produced by
fermentation of moulds such as Penicillium notatum and P. chrysogenum. The most important examples are
benzylpenicillin (penicillin G) and phenoxy-methylpenicillin (penicillin V).
Semisynthetic.
In 1959, scientists at Beecham Research Laboratories succeeded in isolating the penicillin ‘nucleus’, 6-aminopenicillanic acid (6-APA; Figure 11.1A: R represents H). During the commercial production of benzylpenicillin, phenylacetic (phenylethanoic) acid (C6H5.CH2.COOH) is added to the medium in which the Penicillium mould is growing. This substance is a precursor of the side chain (R; see Figure 11.2) in benzylpenicillin. Growth of the organism in the absence of phenylacetic acid led to the isolation of 6-APA; this has a different RF value from benzylpenicillin, which allowed it to be detected chromatographically.
A second method of producing 6-APA came
with the discovery that certain microorganisms produce enzymes, penicillin
amylases (amylases), which catalyse the removal of the side chain from
benzylpenicillin (Figure 11.1B).
Acylation of 6APA with appropriate substances results in new penicillins being
produced which differ only in the nature of the side chain (Table 11.2; Figure 11.2).
Some of these penicillins have considerable activity against Gram-negative as
well as Gram-positive bacteria, and are thus broad-spectrum antibiotics.
Pharmacokinetic properties may also be altered.
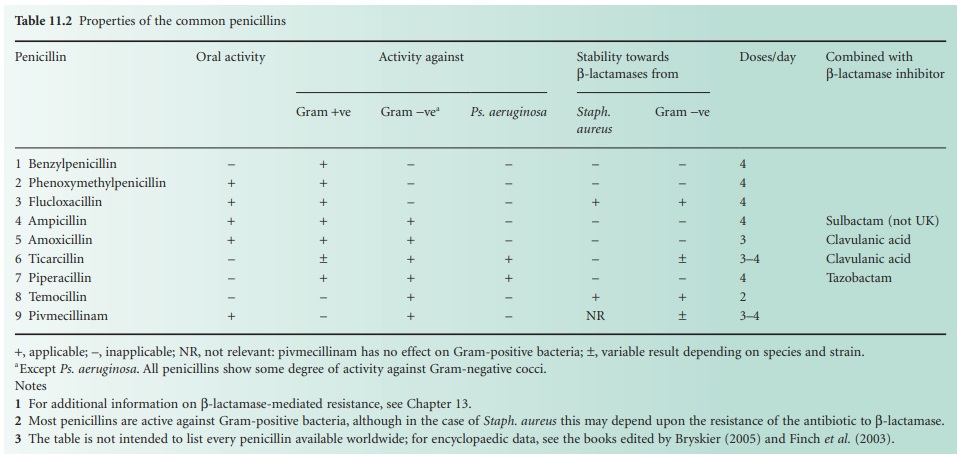
For additional information on β-lactamase-mediated resistance.
Most penicillins are active against
Gram-positive bacteria, although in the case of Staph.
aureus this may depend upon the resistance of the antibiotic to
β-lactamase.
The table is not intended to list
every penicillin available worldwide; for encyclopaedic data, see the books
edited by Bryskier (2005) and Finch et al. (2003).
Except Ps. aeruginosa. All penicillins show some degree of
activity against Gram-negative cocci.
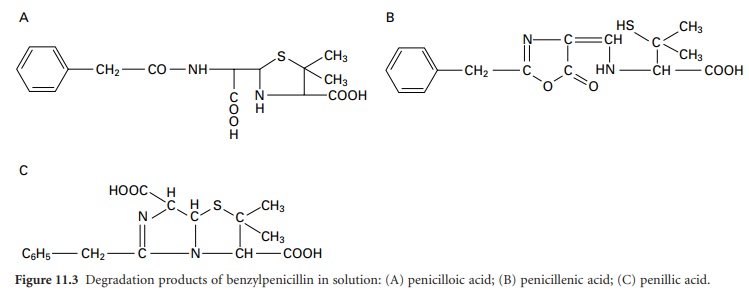
The sodium and potassium salts are very
soluble in water, but they are hydrolysed in solution at a
temperature-dependent rate to the corresponding penicilloic acid (Figure 11.3A)
which is not antibacterial. Penicilloic acid is produced at alkaline pH or (via
penicillenic acid; Figure 11.3B)
at neutral pH, but at acid pH a molecular rearrangement occurs, giving penillic
acid (Figure 11.3C).
Susceptibility to hydrolysis means that penicillins cannot be formulated as
aqueous products, so oral syrups and mixtures must be manufactured as dry
granules for resuspension in water, and injections freeze dried in vials or
ampoules. Typically, aqueous solutions of penicillins lose 10% or more of their
activity in 24 hours at room temperature. Instability in acid medium logically
precludes oral administration, since the antibiotic may be destroyed in the
stomach; for example at pH 1.3 and 35°C benzylpenicillin has a half-life of
less than 5 minutes and is therefore not administered orally, whereas
ampicillin, with a half-life of 600 minutes, is obviously suitable for oral
use. Benzylpenicillin is also rapidly excreted, but this can be overcome by the
use of sparingly soluble salts (benzathine, benethamine and procaine) which
slowly release penicillin into the circulation over a period of time, thus
giving a continuous high concentration in the blood.
Many bacteria produce enzymes,
β-lactamases (formerly called penicillinase), which may inactivate a penicillin
by opening the β-lactam ring, as in Figure 11.1C.
The only clinically significant β-lactamase produced by a Grampositive species
is that of Staph. aureus, but most, if not
all, Gram-negative bacteria have the potential to produce the enzymes, albeit in
such small amounts in some cases that they are of no clinical importance. The
Gram-negative β-lactamases exhibit small interspecies, and even interstrain,
differences in chemical structure which can have profound effects on their
ability to hydrolyse the various β-lactam antibiotics. Some penicillins (Table 11.2)
are considerably more enzymeresistant than others, and consequently may be
extremely valuable in the treatment of infections caused by
β-lactamase-producing bacteria.
The older penicillins, benzylpenicillin
and phenoxymethylpenicillin, are primarily active against Gram-positive bacteria;
they are also β-lactamase sensitive. These shortcomings were overcome to
varying degrees by the semisynthetic penicillins developed in the 1960s.
Ampicillin remained β-lactamase sensitive, but its possession of an amino group
on the benzyl side chain gave the molecule a much broader spectrum of activity
than its parent, benzylpenicillin. The oral absorption of ampicillin was found
to be adequate rather than good; a figure of less than 50% is commonly quoted.
This was significantly improved by the inclusion of a p-hydroxyl group on the benzene ring of the ampicillin
side chain, thus creating amoxicillin, which has largely superseded the older
antibiotic. Both ampicillin and amoxicillin are effective against many
Gram-negative bacteria including Haemophilus influenzae,
Escherichia coli, Salmonella, Shigella and Proteus species, though not Pseudomonas aeruginosa. This last organism has
represented a problem in antibiotic therapy for many years, and carbenicillin,
also developed in the early 1960s, was the first penicillin showing antipseudomonal
activity, although it has now been largely replaced by ticarcillin.
Piperacillin, an acyl derivative of ampicillin, also possesses activity
against Pseudomonas, and, like ticarcillin, is moderately
susceptible to β-lactamases, so both antibiotics are normally used as
combination products with β-lactamase inhibitors. The property of resistance to
Gram-negative β-lactamases, strongly exhibited by temocillin, is conferred by
the possession of a 6α-methoxy group,although this causes the molecule to
exhibit an antibacterial spectrum confined almost exclusively to Gram-negative
species. A similar spectrum arises with pivmecillinam, an amidino-penicillin,
but in this case the enzyme resistance is much weaker.
Penicillins possess a carboxylic acid group on C3 which can be
esterified to create lipophilic prodrugs with enhanced absorption from the
gastrointestinal tract, after which tissue esterases hydrolyse the ester to
release the active antibiotic. This strategy has been particularly successful
in remedying the poor oral absorption of ampicillin and resulted in the
development of bacampicillin, pivampicillin and talampicillin; the first two of
these, in particular, are widely available elsewhere, but not currently in the
UK.
The problem of sensitivity to
staphylococcal β-lactamase was overcome by the development of meticillin in
which the bulky substituent groups of the side chain largely prevented enzyme
binding. Meticillin, which was only available as an injection, has largely been
replaced with other β-lactamase-stable penicillins, particularly the orally
active flucloxacillin, although there are several related drugs whose
availability varies from country to country. The great majority of Staph. aureus strains remained sensitive to
meticillin for about 15 years after its introduction in 1960, but the emergence
of MRSA gathered momentum from the mid 1970s; the incidence in the USA was 2.4%
in 1975 but rose to 35% by 1996.
Penicillins generally are of low toxicity, with allergic reactions as
the only serious problem; these arise more commonly with benzylpenicillin and
ampicillin than the rest. All penicillins, but particularly those administered
orally, can cause diarrhoea, and, rarely, pseudomembranous colitis; this is
more of a problem with ampicillin because a higher proportion of an oral dose
remains in the colon to disturb the natural flora. Penicillins are excreted
primarily in the urine, in which they achieve much higher levels than in the
blood, and accumulation of sodium and potassium may arise with high-dose
injections in patients with poor kidney function.
Related Topics
