Diffusion: Drug transport across a polymeric barrier
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Biopharmaceutical considerations
Drug transport through a polymeric or biological barrier may occur by simple molecular permeation known as molecular diffusion or by move-ment through pores and channels known as pore diffusion.

Diffusion
Drug transport
across a polymeric barrier
Drug
transport through a polymeric or biological barrier may occur by simple
molecular permeation known as molecular
diffusion or by move-ment through pores and channels known as pore diffusion (Figure
4.1).
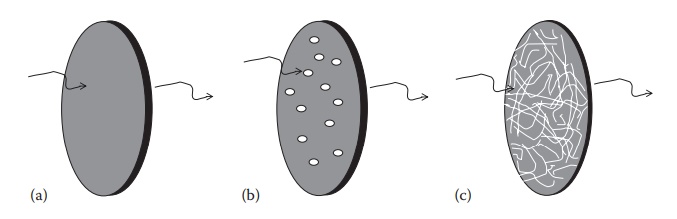
Figure 4.1 An illustration of passive diffusion processes. (a) Diffusion through the homo-geneous film, (b) diffusion through solvent (usually water)-filled pores, and (c) diffusion through and/or between the fibrous membrane strands.
Molecular diffusion
The
transport of a drug molecule through a polymeric membrane that involves
dissolution of the drug in the matrix of the membrane, followed by its
diffusive transport to the surrounding bulk liquid, is an example of simple
molecular diffusion (Figure 4.1a). The release
rate of drug by diffusive transport through the polymeric matrix depends on the
size and
Pore diffusion
Pore
diffusion involves passage of drug through the solvent-filled pores in the
polymeric membrane (Figure 4.1b). In pore
diffusion, the release rate of dissolved drug is affected by porosity of the
membrane, pore structure, sur-face functional groups (e.g., hydrophobic or
hydrophilic), tortuosity, and length of pores.
The
molecules may also pass through the tortuous gaps between the over-lapping
strands of the polymer (Figure 4.1c). In the
cases of both molecu-lar diffusion and pore diffusion, the drug must be
available in a dissolved state. This would be the case if the drug product is
formulated as a drug solution in the polymer. If a formulation consists of a
suspension of drug particles in the polymer, another kinetic step of
dissolution of the drug into the polymer or the solvent is involved. The rate
of dissolution of a drug would depend
on the degree of crystallinity, crystal size, and surface area of the drug;
intrinsic dissolution rate of the drug in the polymer and/or the solvent;
degree of swelling of the polymer with the solvent; and the extent of
mechanical agitation in the system. Drug dissolution from its particles will be
discussed in the next section.
Matrix erosion
In
addition to molecular diffusion and pore diffusion, erosion of the poly-meric
matrix may often be involved in the case of biodegradable polymers. The kinetic
contribution of matrix erosion to the drug release rate would depend on the
relative rates of drug dissolution, polymer erosion, drug dis-solution in the
polymer, drug dissolution in the bulk solvent, molecular diffusion, and pore
diffusion.
Related Topics