Fluid Flow
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Fluid Flow
Fluid flow is an essential element of many pharmaceutical processes.
Fluid Flow
INTRODUCTION
Fluid
flow is an essential element of many pharmaceutical processes. The ability to
propel fluids through pipes and to direct materials from one location to
another is central to the successful manufacture of many products. Fluids
(liq-uids and gases) are a form of matter that cannot achieve equilibrium under
an applied shear stress but deform continuously, or flow, as long as the shear
stress is applied.
Viscosity
Viscosity
is a property that characterizes the flow behavior of a fluid, reflecting the
resistance to the development of velocity gradients within the fluid. Its
quantitative significance may be explained by reference to Figure 2.1. A fluid
is contained between two parallel planes each of area A m2 and
distance h m apart. The upper plane is subjected to a shear force of F N and
acquires a velocity of u m/sec relative to the lower plane. The shear stress,
t, is F/A N/m2. The velocity gradient or rate of shear is given by u/h or, more generally, by the
differential coefficient du/dy, where
y is a distance measured in a
direction perpendicular to the direction of shear. Since this term is described
by the units velocity divided by a length, it has the dimension T-1
or, in this example, reciprocal seconds. For gases, simple liquids, true
solutions, and dilute disperse systems, the rate of shear is proportional to
the shear stress. These systems are called Newtonian, and we can write
F/A = t = η du/dy (2:1)
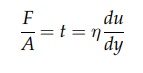
The
proportionality constant η is the dynamic viscosity of the fluid: the higher
its value, the lower the rates of shear induced by a given stress. The
dimensions of dynamic viscosity are M L-1 T-1. For the SI
system of units, viscosity is expressed in N·sec/m2. For the
centimeter-gram-second (CGS) sys-tem, the unit of viscosity is poise (P). One
N·sec/m2 is equivalent to 10 P. The viscosity of water at room
temperature is about 0.01 P or 1 centipoise (cP). Pure glycerin at this
temperature has a value of about 14 P. Air has a viscosity of 180 x 10-6
P.
Complex
disperse systems fail to show the proportionality described by equation (2.1),
the viscosity increasing or, more commonly, decreasing with increase in the
rate of shear. Viscosity may also depend on the duration of shear and even on
the previous treatment of the fluids. Such fluids are termed non-Newtonian.
Equation
(2.1) indicated that wherever a velocity gradient is induced within a fluid, a
shear stress will result. When the flow of a fluid parallel to some boundary is
considered, it is assumed that no slip occurs between the boundary and the
fluid, so the fluid molecules adjacent to the surface are at rest (u = 0). As
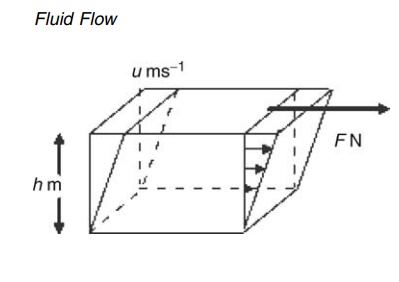
FIGURE 2.1 Schematic of fluid flow depicting the applied force, velocity in the
direction of motion, and thickness of the fluid.
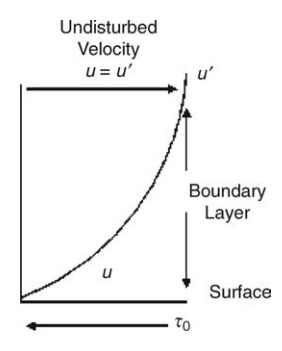
FIGURE 2.2 Distribution of velocities at a boundary layer.
shown in Figure 2.2,
the velocity gradient du/dy decreases
from a maximum at the boundary (y = 0) to zero at some distance from
the boundary (y = y’) when the velocity
becomes equal to the undisturbed velocity of the fluid (u = u’). The shear stress must, therefore,
increase from zero at this point to a maximum at the boundary. A shear stress,
opposing the motion of the fluid and sometimes called fluid friction, is
therefore developed at the boundary. The region limited by the dimension y’, in which flow of
the fluid is perturbed by the boundary, is called the boundary layer. The
structure of this layer greatly influences the rate at which heat is
transferred from the boundary to the fluid under the influence of temperature
gradient or the rate at which molecules diffuse from the boundary into the
fluid under a concentration gradient. These topics are discussed in chapters 3
and 4.
Compressibility
Deformation
is not only a shear-induced phenomenon. If the stress is applied normally and
uniformly over all boundaries, then fluids, like solids, decrease in volume.
This decrease in volume yields a proportionate increase in density. Liquids can
be regarded as incompressible, and changes of density with pres-sure can be
ignored, with consequent simplification of any analysis. This is not possible
in the study of gases if significant changes in pressure occur.
Surface Tension
Surface
tension, a property confined to a free surface and therefore not applicable to
gases, is derived from unbalanced intermolecular forces near the surface of a
liquid. This may be expressed as the work necessary to increase the surface by
unit area. Although not normally important, it can become so if the free
surface is present in a passage of small-diameter orifice or tube. Capillary
forces, determined by the surface tension and the curvature of the surface, may
then be comparable in magnitude to other forces acting in the fluid. An example
is found in the movement of liquid through the interstices of a bed of porous
solids during drying.
Related Topics
