Pharmaceutical Process Engineering
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Introduction
Process engineering has been a central activity in pharmaceutical product development since its inception.
Introduction
Process
engineering has been a central activity in pharmaceutical product development
since its inception. The knowledge that key fundamentals and the processes to
which they are applied were in most respects equivalent to those in other
industries allowed chemical and mechanical engineering principles to be
adopted, which were thoroughly understood (McCabe et al., 1997).
The
first chapters of this book describe in some detail the important fundamentals
of fluid flow, heat and mass transfer. The intent is not to duplicate the
authority of an engineering text of which there are many to which this volume
owes tribute. Rather it is hoped that the topics are covered in sufficient
depth to allow professionals in the pharmaceutical sciences, without engineering
training, to feel comfortable when faced with matters pertaining to these
topics. The chapters immediately following the fundamentals introduce processes
that either employ the principles described earlier or that relate to important
aspects of pharmaceutical development. Hence, these might be considered methods
of handling and conveying different states of matter, that is, solids, liquids,
or gases. The heterogeneous nature of many pharmaceutical formulations leads to
a degree of empiricism in the understanding of processes and their application
to achieve the desired goals of uniform and reproducible drug delivery from the
designated dosage form or the handling of environ-mental or other conditions
related to their preparation. Consequently, the chapters dealing with solids
(including crystallization, powders, size, mixing, and blending), filtration,
sterilization, evaporation, drying, and humidity have their basis in theory but
often invoke semiempirical interpretation. Figure 1.1 illustrates the relationship
between the various unit processes and the under-lying fundamental principles.
The
value of any text in pharmaceutical process engineering is that the fundamental
nature of the topics presented gives it a long shelf life since the science and
engineering have not changed significantly in decades. However, the last decade
has been characterized by changes in the common practices of conducting
experiments to rapidly and efficiently define the process accom-panied by a
change in the regulatory environment in which manufacturing is conducted.
It
may seem premature to introduce this evolving technical and regulatory
consideration into an otherwise slowly changing foundational text. However, it
appears that the important principles of statistical experimental design, risk
assessment, and quality by design, including specific tools to aid with these
approaches, are established elements of process engineering.
The
final chapters of the book relate to these topics. In the information age, the
advent of computer technology allows the collection of vast quantities of data,
which can then be manipulated in real time or near real time to promote the
quality of the product and to ultimately bring therapies to patients. The
challenge of working in this environment is to manipulate this data to fulfill
the promise shown in Figure 1.2.
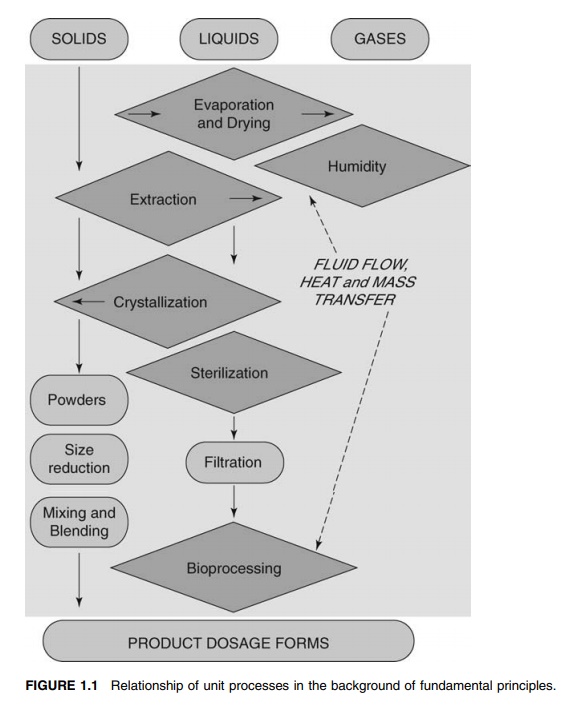
FIGURE 1.1 Relationship of unit processes
in the background of fundamental principles.
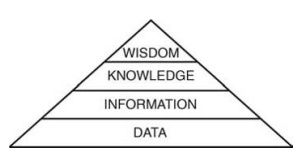
FIGURE 1.2 Schematic of levels of under-standing that emanate from a comprehen-sive base of data.
The intent is to
derive from an extensive database crucial information that increases the body
of knowledge of the process or product and ultimately allows the wise
intervention to bring about a desired objective. This may seem self-evident,
but it could be argued that until relatively recently insufficient data could
be acquired to adequately elevate our understanding through the upper levels of
intelligent management. The practicalities of the experiments and their conduct
in a regulated environment may not differ dramatically from previous periods in
history, but the consideration of an operating framework and the facility to
acquire relevant data has changed substantially. This is undoubtedly an
improvement and should be embraced by all to elevate activities to a higher
level of control and prediction commensurate with a 21st century industry.
In this context, the
final chapters of the book cover in some detail the basis for statistical
experimental design, risk assessment, and supporting tools of process
analytical technology associated with quality by design. Figure 1.3 illustrates
the collation of input variables that is required to predict and control the
output for any process.
If
successful in this endeavor, the cost and efficiency of processes in the future
may be managed by informed decisions that facilitate rapid product development.
In broad terms, the following sections, therefore, consider: (i) fundamental
principles; (ii) unit processes; and (iii) experimental design, data
management, and interpretation. The intent is to begin to address process
engineering in a quality systems environment.
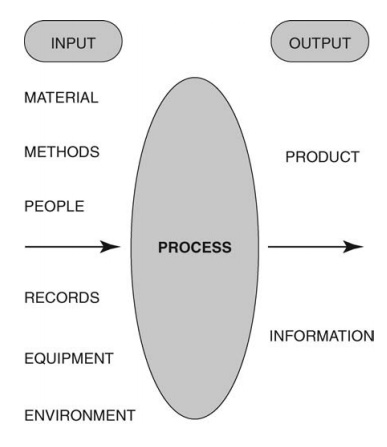
FIGURE 1.3 Process
input variables and their contribution to output properties.
Related Topics
