Transdermal Drug Delivery Systems
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Controlled Release Medication
Transdermal delivery systems are topically administered medicaments in the form of patches that deliver drugs for systemic effects at a predetermined and controlled rate.
TRANSDERMAL DRUG DELIVERY SYSTEMS
Transdermal delivery systems are topically
administered medicaments in the form of patches that deliver drugs for systemic
effects at a predetermined and controlled rate. Some of the advantages of these systems over other
controlled-release formulations are:
1. Drugs with very short
half-lives e.g. nitroglycerine when administered as transdermal patches,
release medicaments at a constant rate for a time period more than that
obtainable with oral formulations.
2. Drugs with narrow therapeutic
indices can be safely administered since better control of release is possible.
3. The noninvasive nature of
these systems permits easy removal and termination of drug action in situations
of toxicity.
4. Problems encountered with oral
administration like degradation, gastric irritation, first-pass effect, etc.
are avoided.
The route is unsuitable
when —
4. Drug dose is large
5. Drug has large molecular size
(makes absorption difficult; should ideally be below 800-1000)
6. Drug is skin sensitising and
irritating
7. Drug is metabolized in skin
8. Drug undergoes protein binding
in skin
9. Drug is highly lipophilic or
hydrophilic (should be moderately soluble in both oil and water).
Other disadvantages
of such systems include variation in absorption efficiency at different sites
of skin, difficulty of adhesion to certain skin types and length of time for
which a patch can be left on any area due to permeability changes (usually not
more than 7 to 10 days).
Several types
of transdermal drug delivery devices are available but they can be basically
divided into two broad categories based on the mechanism by which drug release
is controlled:
1. Monolithic (or matrix)
systems.
2. Reservoir (or membrane)
systems.
All such devices are fabricated as multilayer
laminate structures in which the drug-polymer matrix or a drug reservoir is
sandwiched between two polymeric layers. The outer layer, called as backing layer, is impermeable and meant
to prevent drug loss through it. It is generally composed of metallized
plastic. The other layer which contacts the device with the skin is adhesive layer. It is permeable and in
some cases, may act as rate-limiting membrane. It is generally made of pressure sensitive adhesive materials
like acrylic copolymers, polyisobutylene and polysiloxane or contact adhesives.
The choice
of monolithic or reservoir type of system for controlling drug release depends
upon the major rate-limiting step in the absorption of drug from such devices.
The two rate-limiting steps are:
1. Rate of drug diffusion from
the device, R1, and
2. Rate of drug permeation
through the stratum corneum, R2.
The overall rate of drug transport is proportional
to the sum (R1 + R2).
Monolithic (or Matrix) Devices
These devices are used when R2 is the
rate-controlling step (R2 <
R1) and the drug has a
large therapeutic index so that overdosing does not precipitate toxic
reactions. The two categories of
matrix devices are—one in which the drug is dissolved (usually below saturation
levels) in the polymer matrix and the other in which the drug is dispersed
(generally much above saturation levels). The polymers employed for matrix
systems may be hydrophilic or lipophilic and includes PVC, PVP, polysaccharides,
polyesters, microporous polypropylene and ethylene vinyl acetate copolymers.
The drug release rate from matrix systems is rapid
Rate Controlling Factors :
Drug concentration in
olymer matrix
Chemical nature of
polymer matrix
Geometry of device
Fig. 14.18 Schematic representation of a monolith (matrix) transdermal drug
delivery system
initially and falls as the matrix gets depleted of
drug. The rate is thus proportional to the square root of time. Fig. 14.18.
shows a typical matrix system.
Reservoir (or Membrane) Devices
These devices are used when drug permeation rate is
rapid and absorption should therefore be controlled by controlling drug release
(R1 < R2). It is also suitable for potent drugs with
low therapeutic indices where monitoring drug levels in a narrow range is
essential. The drug is usually
Rate Controlling Factors :
Membrane thickness
Membrane permeability
Fig. 14.19 Schematic representation of reservoir (membrane) type of transdermal
drug delivery system
contained within the reservoir as a suspension in a
liquid (such as silicone) or gel carrier. The rate-controlling thin polymeric
membrane is made of olefinic polymers and copolymers, cellulosic esters, polyamides
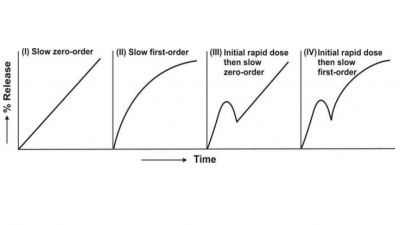
or PVC. When applied on skin, the device shows a rapid release at first
(initial burst effect) followed by a constant zero-order release as long as the
solution inside the reservoir is saturated. Fig. 14.19 shows such a device.
Mixed Monolithic-Reservoir Devices
A third type of system, it is basically a device
having drug release kinetics intermediate between monolithic and reservoir
systems. Here the drug-polymer matrix is layered by a rate-controlling
membrane. Release is controlled initially by the membrane but as the drug gets
depleted, the rate is controlled by diffusion of drug through a thicker layer
of polymer matrix.
A major limitation of transdermal therapy is poor
skin penetrability of several drugs. This problem can be overcome by use of penetration enhancers such as glycerol,
propylene glycol, DMSO, SLS, etc.
Drugs commonly presented in such systems are
nitroglycerine, clonidine, scopolamine and estradiol.
Related Topics