Oral Controlled Release Systems
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Controlled Release Medication
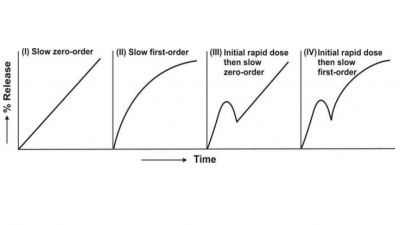
The controlled-release systems for oral use are mostly solids and based on dissolution, diffusion or a combination of both mechanisms in the control of release rate of drug. Depending upon the manner of drug release, these systems are classified as follows:
ORAL CONTROLLED RELEASE SYSTEMS
Oral route has been the most popular and
successfully used route for controlled delivery of drugs because of convenience
and ease of administration, greater flexibility in dosage form design (possible
because of versatility of GI anatomy and physiology) and ease of production and
low cost of such a system.
The controlled-release systems for oral use are
mostly solids and based on dissolution, diffusion or a combination of both
mechanisms in the control of release rate of drug. Depending upon the manner of
drug release, these systems are classified
as follows:
A. Continuous Release Systems: These
systems release the drug for a prolonged
period of time along the entire length of GIT (especially up to the
terminal region of small intestine) with normal transit of the dosage form. The
various systems under this category are:
·
Dissolution controlled release
systems
·
Diffusion controlled release
systems
·
Dissolution and diffusion
controlled release systems
·
Ion-exchange resin-drug complexes
·
Slow dissolving salts and complexes
·
pH-dependent formulations
·
Osmotic pressure controlled
systems
·
Hydrodynamic pressure controlled
systems
B. Delayed Transit and Continuous Release Systems: These systems are designed to
prolong their residence in the GIT along with their release. Often, the dosage
form is fabricated to detain in the stomach and hence the drug present therein
should be stable to gastric pH. Systems included in this category are:
·
Altered density systems
·
Mucoadhesive systems
·
Size-based systems
C. Delayed Release Systems: The
design of such systems involve release of drug only at a specific site in the GIT. The drugs contained in such a
system are those that are:
·
Destroyed in the stomach or by
intestinal enzymes
·
Known to cause gastric distress
·
Absorbed from a specific intestinal
site, or
·
Intended to exert local effect at
a specific GI site.
The two types of delayed release systems are:
·
Intestinal release systems
·
Colonic release systems
Dissolution Controlled Release Systems
Such systems are easiest to design. The drug
present in such a system may be the one:
·
With inherently slow dissolution
rate e.g. griseofulvin and digoxin; such drugs act as natural prolonged release
products
·
That produce slow dissolving
forms when it comes in contact with GI fluids e.g. ferrous sulphate, or
·
Having high aqueous solubility
and dissolution rate e.g. pentoxifylline.
Drugs belonging to the last category present
challenge in controlling their dissolution rate. The techniques employed are:
·
Embedment in slowly dissolving or
erodible matrix, and
·
Encapsulation or coating with
slowly dissolving or erodible substances (Fig. 14.6.).
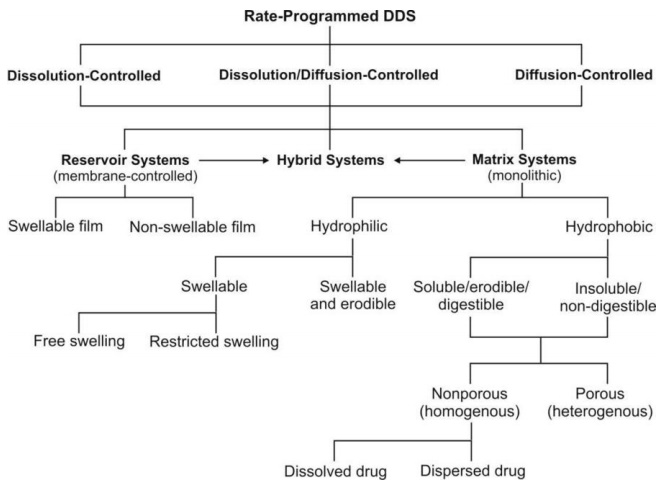
Fig. 14.6 Schematic representation of dissolution controlled release systems matrix system, and (b) coated/encapsulated system
Matrix (or Monolith) Dissolution Controlled Systems
Matrix systems are also called as monoliths since the drug is
homogeneously dispersed throughout a rate-controlling medium. They are very
common and employ waxes such as beeswax, carnauba wax, hydrogenated castor oil,
etc. which control drug dissolution by controlling the rate of dissolution
fluid penetration into the matrix by altering the porosity of tablet,
decreasing its wettability or by itself getting dissolved at a slower rate. The
wax embedded drug is generally prepared by dispersing the drug in molten wax
and congealing and granulating the same. The drug release is often first-order
from such matrices.
Encapsulation/Coating Dissolution Controlled Systems (Reservoir Devices)
Here, the drug particles are coated or encapsulated
by one of the several microencapsulation techniques with slowly dissolving
materials like cellulose, PEGs, polymethacrylates, waxes, etc. The resulting
pellets may be filled as such in hard gelatin capsules (popularly called as spansules) or compressed into tablets.
The dissolution rate of coat depends upon the solubility and thickness of the
coating which may range from 1 to 200 microns.
Diffusion Controlled Release Systems
In these types of systems, the rate-controlling step
is not the dissolution rate but the diffusion of dissolved drug through a
polymeric barrier. The drug release rate is never zero-order since the
diffusional path length increases with time as the insoluble matrix is
gradually depleted of drug. The two types of diffusion controlled systems
are—matrix systems and reservoir devices.
Matrix Diffusion Controlled Systems
Here, the drug is dispersed in an insoluble matrix of rigid non-swellable hydrophobic materials or swellable hydrophilic substances. Materials used for rigid matrix are insoluble plastics such as PVC and fatty materials like stearic acid, beeswax, etc. With plastic materials, the drug is generally kneaded with the solution of PVC in an organic solvent and granulated. Waxy matrix is prepared by dispersing the drug in molten fat followed by congealing. The granules are then compressed into tablets (Fig. 14.7.a). Swellable matrix systems are popular for sustaining the release of highly water-soluble drugs. The material for such matrices are generally hydrophilic gums and may be of natural origin (guar gum, tragacanth), semisynthetic (HPMC, CMC, xanthan gum) or synthetic (polyacrylamides). The drug and the gum are granulated together with a solvent such as alcohol and compressed into tablets. The release of drug from such initially dehydrated hydrogels involves simultaneous absorption of water (resulting in hydration, gelling and swelling of gum) and desorption of drug via a swelling controlled diffusion mechanism. As the gum swells and the drug diffuses out of it, the swollen mass, devoid of drug appears transparent or glasslike and therefore the system is sometimes called as glassy hydrogel (Fig. 14.7b).
Rate Controlling Step :
Diffusion of dissolved drug through the matrix
Fig. 14.7 Diffusion controlled devices—(a) rigid matrix, and (b) swellable matrix
The drug release follows Fickian
first-order diffusion under equilibrium conditions. However, during the
swelling process, such an equilibrium may not exist and the diffusion may be
non-Fickian or anomalous diffusion.
Reservoir Devices (or Laminated Matrix Devices)
These systems are hollow containing an inner core of drug surrounded in a water insoluble polymer membrane. The polymer can be applied by coating or microencapsulation techniques. The drug release mechanism across the membrane involves its partitioning into the membrane with subsequent release into the surrounding fluid by diffusion (Fig. 14.8). The polymers commonly used in such devices are HPC, ethyl cellulose and polyvinyl acetate. A disadvantage of all such microencapsulated drug release systems is a chance of sudden drug dumping which is not common with matrix devices.
Rate Controlling Factors:
Polymeric content in coating,
Thickness of coating,
Hardness of microcapsule
Fig. 14.8 Drug release by diffusion across the insoluble membrane of reservoir
device
Dissolution and Diffusion Controlled Release Systems
In such systems, the drug core is encased in a
partially soluble membrane. Pores are thus created due to dissolution of parts
of the membrane which:
·
Permit entry of aqueous medium
into the core and hence drug dissolution, and
·
Allow diffusion of dissolved drug
out of the system (Fig. 14.9).
An example of obtaining such a coating is using a
mixture of ethyl cellulose with PVP or methyl cellulose; the latter dissolves
in water and creates pores in the insoluble ethyl cellulose membrane.
Rate Controlling Factor : Fraction
of soluble polymer in the coat
Fig. 14.9 Dissolution and diffusion controlled release system
Ion-Exchange Resin-Drug Complexes
Controlled delivery of ionisable acidic and basic
drugs can be obtained by complexing them with insoluble nontoxic anion exchange
and cation exchange resins respectively. The drug is released slowly by
diffusion through the resin particle structure. The following equation
represents the release of a basic drug, NH2R', from a cation
exchange resin RSO3H when in contact with GI fluid containing an
ionic compound A+B- (either gastric HCl or intestinal
NaCl):
RSO3–NH3+R'
+ A+B– → RSO3-A+ + NH3+R'B–
A number of basic drugs like noscapine,
phenylpropanolamine and phentermine have been retarded by such an approach. The
complex can be prepared by incubating the drug-resin solution or passing the
drug solution through a column containing ion-exchange resin. The drug-resin
complex can be coated with cellulose or hard paraffin and formulated as ion
free suspension for paediatric use.
Slow Dissolving Salts and Complexes
Salts or complexes of drugs which are slowly
soluble in the GI fluids can be used for controlled-release of the active
principle. Amine drugs can be reacted with tannic acid to form poorly soluble
complexes that can be formulated as long acting tablets. Penicillin G has been
complexed with N,N’-dibenzyl ethylenediamine to give benzathine penicillin G
that can be formulated as oral suspension. Such complexes can be obtained by
simple acid-base reaction on mixing together solutions of individual compounds.
pH-Independent Formulations
Such systems are designed to eliminate the influence
of changing GI pH on dissolution and absorption of drugs by formulating them
with sufficient amount of buffering agents (salts of phosphoric, citric or
tartaric acids) that adjust the pH to the desired value as the dosage form
passes along the GIT and permit drug dissolution and release at a constant rate
independent of GI pH. The dosage form containing drug and buffer is coated with
a permeable substance that allows entry of aqueous medium but prevents
dispersion of tablet.
Osmotic Pressure Controlled Systems
Unlike the solution-diffusion mechanism for most systems, an oral osmotic pump, popularly called as oros, works on the principle of osmotic pressure to release the drug at a constant zero-order rate. A core comprising of drug and an osmotically active substance (also called as osmogen) such as potassium chloride or mannitol is surrounded by a rigid semipermeable membrane coating such as cellulose ester or cellulose ether having an orifice of 0.4 mm diameter produced by laser beam for drug exit. When exposed to GI fluids, water flows through the semipermeable membrane into the tablet due to osmotic pressure difference which dissolves the drug and pumps it out through the orifice by the osmotic force (Fig. 14.10). Such devices can be used to target specific areas of the GIT.
Rate Controlling Factors :
Orifice diameter
Membrane area
Membrane thickness
Membrane permeability
Osmotic properties of the core
Drug solubility
Fig. 14.10 Oral osmotic pump (oros)
The oros principle can be used to design multiunit dosage forms consisting of
drug core particles coated with a water permeable membrane in which the
delivery orifice is made by using a channelling agent such as PVP and the
coated particles filled in a capsule.
Hydrodynamic Pressure Controlled Systems
The hydrodynamic pressure generated by swelling of a hydrophilic gum can also be used to activate the delivery of drugs. The device comprises of a rigid, shape retaining housing enclosing a collapsible, impermeable compartment containing liquid drug (Fig. 14.11). The space between the external housing and the drug compartment contains a layer of swellable, hydrophilic gum such as polyhydroxyalkyl methacrylate. In the GIT, the gum imbibes water through the opening present at the lower side of external housing and swells creating a hydrodynamic pressure. The pressure thus created squeezes the collapsible drug reservoir to release the medicament through the delivery orifice at a zero-order rate. Such systems are also called as push-pull osmotic pumps.
Rate Controlling Factors :
Fluid permeability
Surface area of wall with openings
Hydrodynamic pressure gradient
Fig. 14.11 Hydrodynamic pressure controlled
system (push-pull osmotic pump)
Altered Density Systems
The transit time of GI contents is usually less
than 24 hours. This is the major limiting factor in the design of oral
controlled-release formulations which can reduce the frequency of dosing to a
time period little more than the residence time of drug. However, if the
residence time of drug in the stomach and/or intestine is prolonged in some
way, the frequency of dosing can be further reduced. There are 3 ways by which
this can be achieved—altering the density of drug particles, use of mucoadhesive
polymers and altering the size of the dosage form. The altered density approach
involves use of either high or low density pellets.
High Density Pellets
The density of GI fluids is around 1.4 g/cc. Use of
drug pellets having density greater than this value, preferably above 1.6 g/cc,
results in prolonged GI residence that is unaffected by food. Iron oxide,
titanium dioxide and barium sulfate have been used to increase the density of
drug pellets. The drug is coated on the heavy core and then covered by a diffusion
controlled membrane (Fig. 14.12a).
Low Density Pellets
Also called as hydrodynamically
balanced systems, such pellets, having density less than that of GI fluids,
float on the gastric juice for an extended period of time while slowly
releasing the drug. Globular shells such as that of poprice and celluloses have
been used to lower the density of system (Fig. 14.12b). A swellable gum like HPMC can be used for a similar purpose
(Fig. 14.12c).
Fig. 14.12 Altered density systems
Floating or buoyant tablets/capsules can be
formulated by granulating a drug with 20
to 80% of hydrogel such as HPMC, HEC and HPC. On contact with GI fluids, the
tablet swells and forms a diffusible gel barrier that lowers the density of
system to less than 1 allowing it to float. Lipophilic polymers such as
silicone elastomer can also be modified to have swelling properties. This is
achieved by impregnating a water miscible liquid such as glycerol or a
water-soluble salt such as sodium chloride in the lipophilic matrix. On contact
with aqueous medium, the modified lipophilic polymer swells due to absorption
of water by the hydrophilic additives in the matrix. Alternatively, a gas
filled flotation chamber can be attached to a membrane coated tablet for making
it buoyant.
Mucoadhesive Systems
A bioadhesive polymer such as cross-linked
polyacrylic acid, when incorporated in a tablet, allows it to adhere to the
gastric mucosa or epithelium. Such a system continuously releases a fraction of
drug into the intestine over prolonged periods of time.
Size-Based Systems
Gastric emptying of a dosage form can be delayed in
the fed state if its size is greater than 2 mm. Dosage form of size 2.5 cm or
larger is often required to delay emptying long enough to allow once daily
dosing. Such forms are however difficult to swallow.
Intestinal Release Systems
A drug may be enteric coated for intestinal release
for several known reasons such as to prevent gastric irritation, prevent
destabilization in gastric pH, etc. Certain drugs are delivered to the distal
end of small intestine for absorption via Peyer’s patches or lymphatic system. Peyer’s patches are mucosal lymphoid
tissues that are known to absorb macromolecules like proteins and peptides and
antigens by endocytosis. Selective release of such agents to Peyer’s patch
region prevents them from getting destroyed/digested by the intestinal enzymes.
Such a site can be utilized for oral delivery of insulin. Lymphatic system on the other hand is known to absorb highly
lipophilic agents directly into the systemic circulation without their
first-pass through liver. The drug is absorbed by two mechanisms—chylomicrons
which are fatty vesicles that entrap hydrophobic drugs, and pinocytic uptake of
macromolecules.
Colonic Release Systems
Drugs are poorly absorbed through colon but may be
delivered to such a site for two reasons—
a. Local action as in the
treatment of ulcerative colitis with mesalamine, and
b. Systemic absorption of protein
and peptide drugs like insulin and vasopressin.
Advantage is taken of the fact that pH-sensitive
bioerodible polymers like polymethacrylates release the medicament only at the
alkaline pH of colon or use of divinylbenzene cross-linked polymers that can be
cleaved only by the azoreductase of colonic bacteria to release free drug for
local effect or systemic absorption.
Related Topics