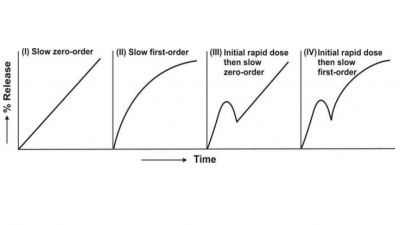
Controlled Release Medication
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Controlled Release Medication
An ideal dosage regimen in the drug therapy of any disease is the one which immediately attains the desired therapeutic concentration of drug in plasma (or at the site of action) and maintains it constant for the entire duration of treatment.
Controlled Release Medication
An ideal dosage regimen in the drug therapy of any disease is the one which immediately attains
the desired therapeutic concentration of drug in plasma (or at the site of
action) and maintains it constant for the entire duration of treatment. This is possible through
administration of a drug delivery system in a particular dose and at a
particular frequency. The term drug
delivery covers a broad range of techniques used to get therapeutic agents
into the human body. The frequency of administration or the dosing interval of
any drug depends upon its half-life or mean residence time (MRT) and its
therapeutic index. When a drug is delivered as a conventional dosage form such
as a tablet, the dosing interval is much shorter than the half-life of the drug
resulting in a number of limitations
associated with such a conventional dosage form:
1. Poor patient compliance;
increased chances of missing the dose of a drug with short half-life for which
frequent administration is necessary.
2. A typical peak-valley plasma concentration-time profile is obtained which
makes attainment of steady-state condition difficult (see Fig. 14.1).
3. The unavoidable fluctuations
in the drug concentration may lead to under-medication
or over-medication as the Css values fall or rise beyond the therapeutic range.
4. The fluctuating drug levels
may lead to precipitation of adverse effects especially of a drug with small
therapeutic index whenever overmedication occurs.
There are two ways to overcome such a situation –
1. Development of new, better and
safer drugs with long half-lives and large therapeutic indices, and
2. Effective and safer use of
existing drugs through concepts and techniques of controlled and targeted
delivery systems.
The first approach has many disadvantages which
therefore resulted in increased interest in the second approach. The second
approach, owing to several technical advancements, has resulted in the development
of drug delivery systems capable of controlling the rate of drug delivery,
sustaining the duration of therapeutic action and/or targeting the delivery of
drug to a particular tissue. An ideal drug delivery system should
deliver the drug at a rate dictated by the needs of the body over a specified
period of treatment. This idealized objective points to the two aspects most
important to drug delivery –
·
Spatial delivery of drug which relates to targeting a drug to a specific
organ or tissue, and
·
Temporal delivery of drug which refers to controlling the rate or specific
time of drug delivery to the target tissue.
An appropriately designed controlled-release
drug-delivery system (CRDDS) can improve the therapeutic
efficacy and safety of a drug by precise temporal and spatial placement in the
body, thereby reducing both the size and number of doses required (see Figure
14.1).
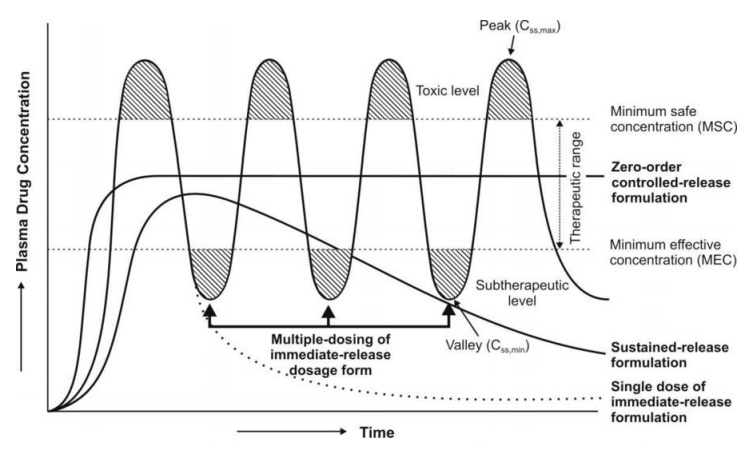
Fig. 14.1 A hypothetical plasma
concentration-time profile from conventional multiple dosing and an ideal controlled delivery formulation
The several advantages
of a controlled drug delivery system over a conventional dosage form are—
1. Improved patient convenience
and compliance due to less frequent drug administration.
2. Reduction in fluctuation in
steady-state levels (Fig. 14.1) and therefore –
·
Better control of disease
condition, and
·
Reduced intensity of local or
systemic side-effects.
3. Increased safety margin of
high potency drugs due to better control of plasma levels.
4. Maximum utilization of drug
enabling reduction in total amount of dose administered.
5. Reduction in health care costs
through –
·
Improved therapy
·
Shorter treatment period
·
Lower frequency of dosing, and
·
Reduction in personnel time to
dispense, administer and monitor patients.
Disadvantages of controlled-release dosage
forms include —
1. Decreased systemic
availability in comparison to immediate-release conventional dosage forms. This
may be due to –
·
Incomplete release
·
Increased first-pass metabolism
·
Increased instability
·
Insufficient residence time for
complete release
·
Site-specific absorption
·
pH-dependent solubility.
2. Poor in vitro–in vivo correlation.
3. Possibility of dose dumping
due to food, physiologic or formulation variables or chewing or grinding of
oral formulations by the patient and thus, increased risk of toxicity.
4. Retrieval of drug is difficult
in case of toxicity, poisoning or hypersensitivity reactions.
5. Reduced potential for dosage
adjustment of drugs normally administered in varying strengths.
6. Higher cost of formulation.
Related Topics