Methods For Enhancement of Bioavailability
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Bioavailability and Bioequivalence
As far as the definition of bioavailability is concerned, a drug with poor bioavailability is the one with
METHODS FOR ENHANCEMENT OF BIOAVAILABILITY
As far as the definition of bioavailability is
concerned, a drug with poor bioavailability is the one with -
·
Poor aqueous solubility and/or slow dissolution rate in biological fluids.
·
Poor permeability through the biomembrane owing to inadequate partition coefficient
or lipophilicity or large molecular size such as that of protein or peptide
drugs such as insulin.
Both solubility as well as permeability of a drug
depends upon its physicochemical characteristics as discussed in Chapter 2.
Based on the intestinal permeability and solubility
of drugs, Amidon et al developed Biopharmaceutics
Classification System (BCS) which classifies the drugs into one of the
4 groups as shown in the table 11.8. The table also shows the approaches
employed to overcome formulation challenges in each class of drugs.
TABLE 11.8.
The Biopharmaceutics Classification System for Drugs
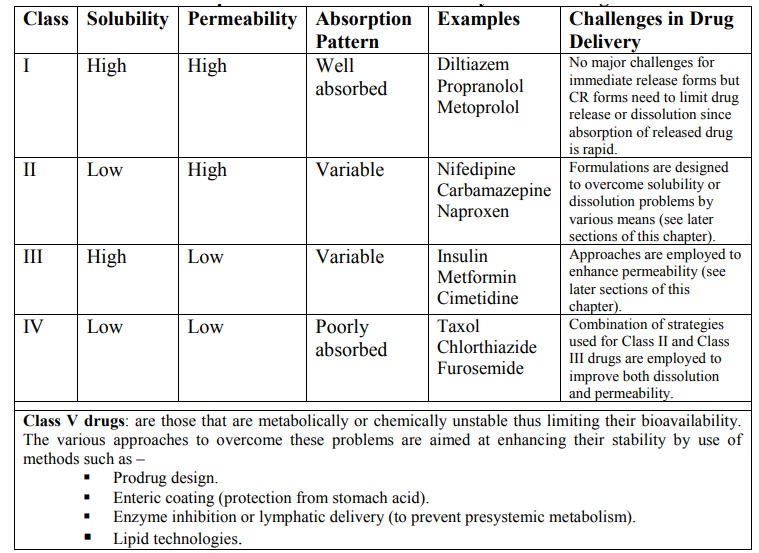
Class V drugs: are those that are
metabolically or chemically unstable thus limiting their bioavailability. The various approaches to overcome
these problems are aimed at enhancing their stability by use of methods such as
–
·
Prodrug design.
·
Enteric coating (protection from
stomach acid).
·
Enzyme inhibition or lymphatic
delivery (to prevent presystemic metabolism).
·
Lipid technologies.
Class I drugs (high solubility/high
permeability) are well absorbed orally since they have neither solubility nor
permeability limitation.
Class II drugs (low solubility/high
permeability) show variable absorption owing to solubility limitation.
Class III drugs (high solubility/low
permeability) also show variable absorption owing to permeability limitation.
Class IV drugs (low solubility/low permeability) are
poorly absorbed orally owing to both solubility and permeability
limitations.
Class V drugs – are the ones that do not come under the purview of BCS classification
but includes drugs whose absorption is limited owing to their poor stability in
GI milieu –
·
Gastric instability (omeprazole).
·
Complexation in GI lumen.
·
First pass metabolism – by intestinal
enzymes (peptide drugs), hepatic enzymes, microbial enzymes, etc.
The BCS classification of a drug depends upon its
three key parameters that control absorption – solubility, dissolution rate and
permeability that correlates with three respective dimensionless parameters –
dose number, dissolution number and absorption number (see table 11.9 and
figure 11.4).
TABLE 11.9.
Drug Properties that Determine BCS Classification
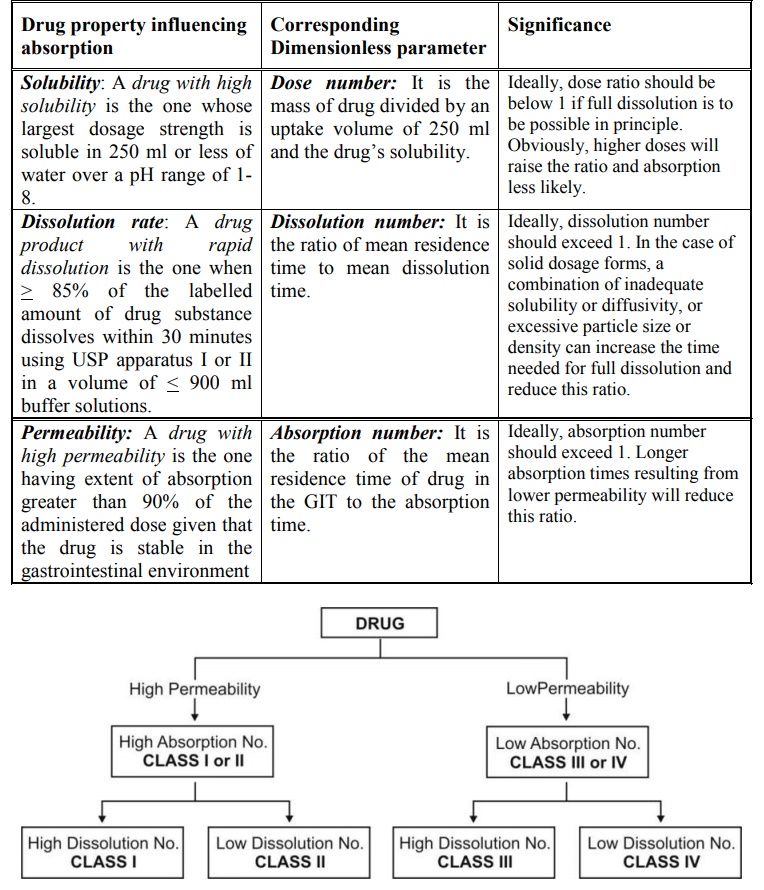
Fig. 11.4 Classification of Drugs on BCS
basis
Solubility Determination – Methods
for determining drug solubility are –
·
pH-solubility profile of test
drug in aqueous media with a pH range of 1 to 7.5.
·
Shake-flask or titration method.
Permeability Determination – The methods are further classified as –
Determination of extent of absorption in humans:
·
Mass-balance pharmacokinetic
studies.
·
Absolute bioavailability studies.
Intestinal permeability methods:
·
In vivo intestinal perfusions studies in
humans.
·
In vivo or in situ intestinal perfusion studies in animals.
·
In vitro permeation experiments with
excised human or animal intestinal tissue.
·
In vitro permeation experiments across
epithelial cell monolayers.
Dissolution Determination – Methods
for determining drug product dissolution are – USP apparatus I
(basket) at 100 rpm or USP apparatus II (paddle) at 50 rpm. Dissolution media
(900 ml): 0.1 N HCl or simulated gastric fluid, pH 4.5 buffer, and pH 6.8
buffer or simulated intestinal fluid.
Compare dissolution profiles of test and reference
products using a similarity factor (f2).
Besides identifying the challenges in formulation
design, the BCS is designed to guide decisions with respect to in vitro and in vivo correlations (IVIVC).
The three conceptual approaches in overcoming the
bioavailability problems of drugs are:
1. The Pharmaceutical Approach which
involves modification of formulation, manufacturing
process or the physicochemical properties of the drug without changing the
chemical structure.
2. The Pharmacokinetic Approach in which
the pharmacokinetics of the drug is altered
by modifying its chemical structure. This approach is further divided into two
categories –
·
Development of new chemical
entity (NCE) with desirable features
·
Prodrug design.
3. The Biological Approach whereby
the route of drug administration may be changed
such as changing from oral to parenteral route.
The second approach of chemical structure
modification has a number of drawbacks of being very expensive and time
consuming, requires repetition of clinical studies and a long time for regulatory
approval. Moreover, the new chemical entity may suffer from another
pharmacokinetic disorder or bear the risk of precipitating adverse effects.
Only the pharmaceutical approach will be dealt herewith.
The pharmaceutical attempts, whether
optimising the formulation, manufacturing process or physicochemical properties
of the drug, are mainly aimed at altering
the biopharmaceutic properties of
drug in one of the several ways –
A. Enhancement of drug solubility
or dissolution rate, as it is the major rate-limiting
step in the absorption of most drugs. This approach applies to class II drugs
according to BCS.
B. Enhancement of drug
permeability. This approach applies to class
III drugs according to BCS.
C. Enhancement of drug stability. This approach applies to class V drugs according to BCS.
D. Enhancement of
gastrointestinal retention. This
approach can apply to class II, III or V drugs.
Related Topics
