Michaelis Menten Equation
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Nonlinear Pharmacokinetics
The kinetics of capacity-limited or saturable processes is best described by Michaelis.
MICHAELIS MENTEN EQUATION
The kinetics of capacity-limited or saturable
processes is best described by Michaelis-
Menten equation:
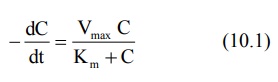
Where,
–dC/dt = rate of decline of drug
concentration with time,
Vmax = theoretical
maximum rate of the process, and
Km = Michaelis
constant.
Three situations can now be considered depending
upon the values of Km and C:
1. When Km = C
Under this situation, the equation 10.1 reduces to:
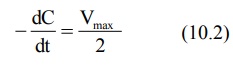
i.e. the rate of process is equal to one-half its
maximum rate (Fig. 10.1).
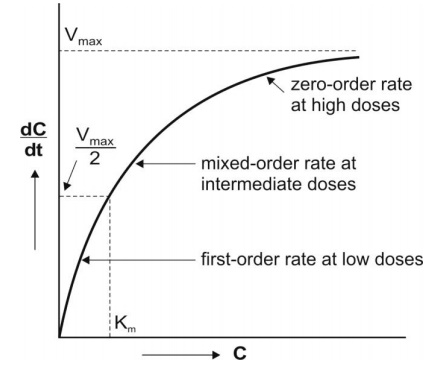
Fig. 10.1 A plot of Michaelis-Menten
equation (elimination rate dC/dt versus concentration
C). Initially, the rate increases linearly (first-order) with concentration,
becomes mixed-order at higher concentration and then reaches maximum (Vmax)
beyond which it proceeds at a constant rate (zero-order).
2. When Km >> C
Here, Km + C ≡ Km and the equation 10.1 reduces to:
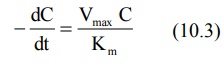
The above equation is identical to the one that
describes first-order elimination of a drug where Vmax/Km
= KE. This means that the drug concentration in the body that results
from usual dosage regimens of most drugs is well below the Km of the
elimination process with certain exceptions such as phenytoin and alcohol.
3. When Km << C
Under this condition, Km + C ≡ C and the equation 10.1 will become:
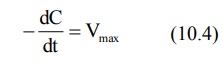
The above equation is identical to the one that
describes a zero-order process i.e. the rate process occurs at a constant rate
Vmax and is independent of drug concentration e.g. metabolism of
ethanol.
Estimation of Km and Vmax
The parameters of capacity-limited processes like
metabolism, renal tubular secretion and biliary excretion can be easily defined
by assuming one-compartment kinetics for the drug and that elimination involves
only a single capacity-limited process.
The parameters Km and Vmax
can be assessed from the plasma concentration-time data collected after i.v.
bolus administration of a drug with nonlinear elimination characteristics.
Rewriting equation 10.1.
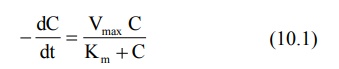
Integration of above equation followed by
conversion to log base 10 yields:
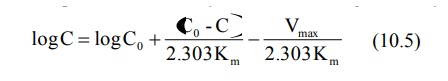
A semilog plot of C versus t yields a curve with a
terminal linear portion having slope –Vmax/2.303Km and
when back extrapolated to time zero gives Y-intercept
log BarC0 (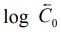 ) (see Fig. 10.2). The equation that
describes this line is:
) (see Fig. 10.2). The equation that
describes this line is:
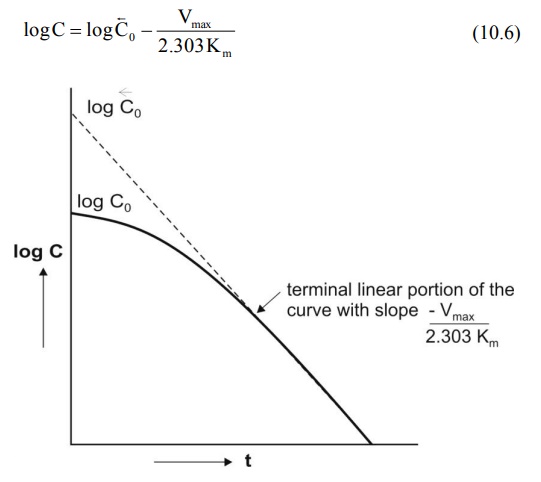
Fig. 10.2 Semilog plot of a drug given as i.v. bolus with nonlinear elimination
and that fits one-compartment
kinetics.
At low plasma concentrations, equations 10.5 and
10.6 are identical. Equating the two and simplifying further, we get:
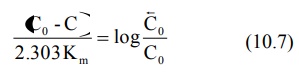
Km can thus be obtained from above equation. Vmax can be computed by substituting the value of Km in the slope value.
An alternative approach of estimating Vmax
and Km is determining the rate of change of plasma drug
concentration at different times and using the reciprocal of the equation 10.1.
Thus:

where Cm = plasma concentration at
midpoint of the sampling interval. A double reciprocal plot or the Lineweaver-Burke plot of 1/(dC/dt)
versus 1/Cm of the above equation yields a straight line with slope
= Km/Vmax and y-intercept = 1/Vmax.
A disadvantage
of Lineweaver-Burke plot is that the points are clustered. More reliable plots
in which the points are uniformly scattered are Hanes-Woolf plot (equation 10.9) and Woolf-Augustinsson-Hofstee plot (equation 10.10).
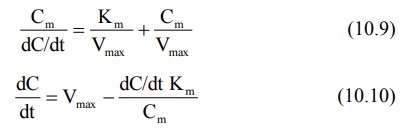
The above equations are rearrangements of equation
10.8. Equation 10.9 is used to plot Cm/(dC/dt) versus Cm
and equation 10.10 to plot dC/dt versus (dC/dt)/C m. The parameters
Km and Vmax can be computed from the slopes and
y-intercepts of the two plots.
Km and Vmax from Steady-State Concentration
When a drug is administered as a constant rate i.v.
infusion or in a multiple dose regimen, the steady-state concentration Css
is given in terms of dosing rate DR
as:
DR = CssClT (10.11)
where DR = Ro when the drug is administered
as zero-order i.v. infusion and it is equal to FXo/τ when administered as multiple oral dosage regimen (F is fraction
bioavailable, Xo is oral dose and is dosing interval).
At steady-state, the dosing rate equals rate of
decline in plasma drug concentration and if the decline (elimination) is due to
a single capacity-limited process (for e.g. metabolism), then;
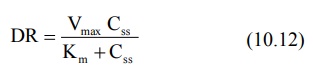
A plot of Css versus DR yields a typical
hockey-stick shaped curve as shown in
Fig. 10.3.
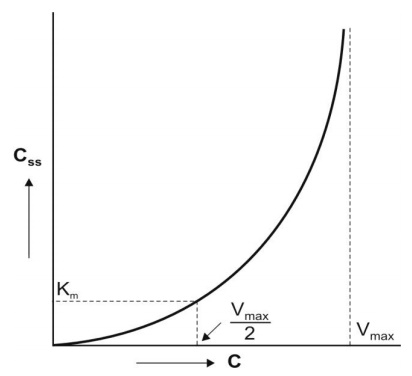
Fig. 10.3 Curve for a drug with nonlinear kinetics obtained by plotting the
steady-state concentration versus
dosing rates.
To define the characteristics of the curve with a
reasonable degree of accuracy, several measurements must be made at steady-state
during dosage with different doses.
Practically, one can graphically compute Km and
Vmax in 3 ways:
1. Lineweaver-Burke Plot/Klotz Plot
Taking reciprocal of equation 10.12, we get:
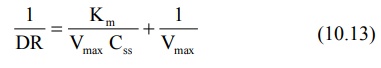
Equation 10.13 is identical to equation 10.8 given
earlier. A plot of 1/DR versus 1/Css yields a straight line with
slope Km/Vmax and y-intercept 1/Vmax
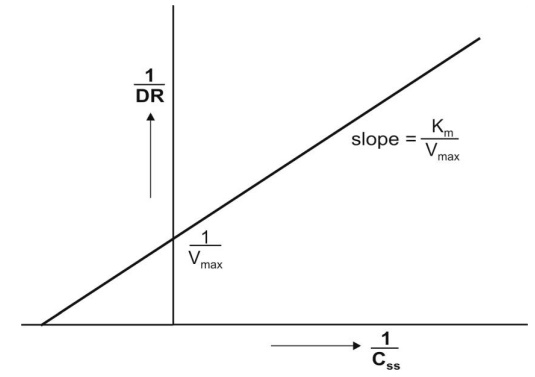
Fig. 10.4 Lineweaver-Burke/Klotz plot for
estimation of Km and Vmax at steady-state
concentration of drug.
2. Direct Linear Plot
Here, the graph is considered as shown in Fig.
10.5. A pair of Css viz. Css,1 and Css,2
obtained with two different dosing rates DR1 and DR2 is
plotted. The points Css,1 and DR1 are joined to form a
line and a second line is obtained similarly by joining Css,2 and DR2.
The point where these two lines intersect each other is extrapolated on DR axis
to obtain Vmax and on x-axis to get Km.
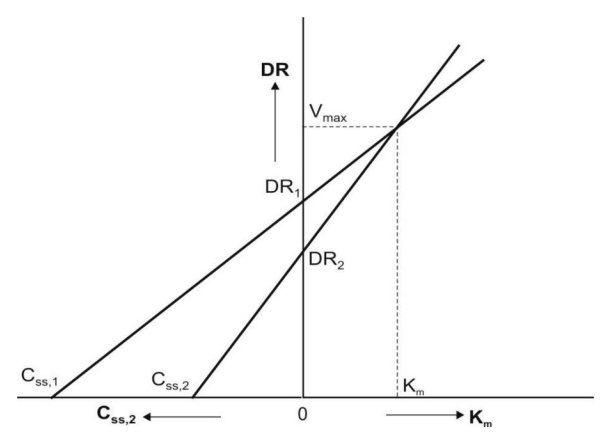
Fig. 10.5 Direct linear plot for estimation of Km and Vmax
at steady-state concentrations of a drug given at different dosing rates.
3. The third graphical method of
estimating Km and Vmax involves rearranging equation 10.12 to yield:
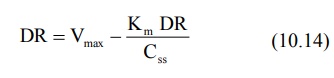
A plot of DR versus DR/Css yields a
straight line with slope -Km and Y-intercept
Vmax.
Km and Vmax can also be
calculated numerically by setting up simultaneous equations as shown below:
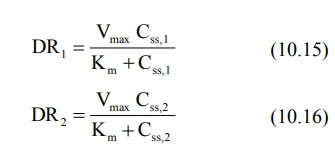
Combination of the above two equations yields:
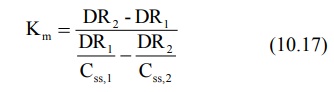
After having computed Km, its subsequent substitution in any one of the two simultaneous equations will yield Vmax.
It has been observed that Km is much
less variable than Vmax. Hence, if mean Km for a drug is
known from an earlier study, then instead of two, a single measurement of Css
at any given dosing rate is sufficient to compute Vmax.
There are several limitations of Km and
Vmax estimated by assuming one-compartment system and a single
capacity-limited process. More complex equations will result and the computed Km
and Vmax will usually be larger when:
1. The drug is eliminated by more
than one capacity-limited process.
2. The drug exhibits parallel
capacity-limited and first-order elimination processes.
3. The drug follows
multicompartment kinetics.
However, Km and Vmax obtained under such circumstances have little practical applications in dosage calculations.
Drugs that behave nonlinearly within the
therapeutic range (for example, phenytoin shows saturable metabolism) yield
less predictable results in drug therapy and possess greater potential in
precipitating toxic effects.
Related Topics
