Micellar solubilization
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Surfactants and micelles
Micelles can be used to increase the solubility of materials that are normally insoluble or poorly soluble in the dispersion medium used. This phenom-enon is known as solubilization, and the incorporated substance is referred to as the solubilizate.
Micellar
solubilization
Micelles
can be used to increase the solubility of materials that are normally insoluble
or poorly soluble in the dispersion medium used. This phenom-enon is known as solubilization, and the incorporated
substance is referred to as the solubilizate.
For example, surfactants are often used to increase the solubility of poorly
soluble steroids. The location, distribution, and orienta-tion of solubilized
drugs in the micelles influence the kinetics and extent of drug solubilization.
These parameters are determined by the molecular loca-tion of the interaction
of drugs with the structural elements or functional groups of the surfactant in
the micelles.
1. Factors affecting the extent of solubilization
Factors
affecting the rate and extent of micellar solubilization include the nature of
surfactants, the nature of solubilizates, temperature, and pH.
1. Nature of
surfactants:
Structural characteristics of a surfactant
affect its solubilizing capacity because of its effect on the
solubiliza-tion site within the micelle. In cases where the solubilizate is
located within the core or deep within the micelle structure, the
solubili-zation capacity increases with increase in alkyl chain length. For
example, there was an increase in the solubilizing capacity of a series of
polysorbates for selected barbiturates as the alkyl chain length was increased
from C12 (polysorbate 20) to C18 (polysorbate 80).
An
increase in the alkyl chain length increases the hydrophobicity of the core and
micellar radius, reduces pressure inside the micelle, and increases the
diffusive entry of the hydrophobic drug into the micelle. In addition, the
solubilization of the poorly soluble drug tropicamide increased with increase
in the oxyethylene content of poloxamer. On the other hand, an increase in the
ethylene oxide chain length of a polyoxyethylated nonionic surfactant led to an
increase in the total amount solubilized per mole of surfactant because of the
increasing number of micelles. Thus, the effect of increase in the number of
micelles of the same (smaller) size can be very different than increase in the
size of micelles.
2. Nature of
solubilizate (drug being solubilized): The location of
solu-bilizates in the micelles is closely related to the chemical nature of the
solubilizate. In general, nonpolar, hydrophobic solubilizates are local-ized in
the micellar core. Compounds that have both hydrophobic and hydrophilic regions
are oriented with the hydrophobic group facing or in the core and the
hydrophilic or polar groups facing toward the sur-face. For a hydrophobic drug
solubilized in a micelle core, an increase in the lipophilicity or the
lipophilic region or surface area of the drug leads to solubilization near the
core of the micelle and enhances drug solubility.
Unsaturated
compounds are
generally more soluble than their satu-rated counterparts. Solubilizates that
are located within micellar core tend to increase the size of the micelles.
Micelles become larger not only because their core is enlarged by the
solubilizate but also because the number of surfactant molecules per micelle
increases in an attempt to cover the swollen core.
3. Effect of
temperature:
In general, the amount of the drug solubilized increases with an increase in temperature (Figure 10.5). The effect is particularly pronounced with some
nonionic surfactants, where it is a consequence of an increase in the micellar
size with increasing temperature.
4. Effect of pH: The main effect of
pH on solubilizing ability of non-ionic surfactants is to alter the equilibrium
between ionized and unionized drugs. The overall effect of pH on drug
solubilization is a function of proportion of ionized and unionized forms of
the drug in solution and in micelles, which is determined by (1) the pKa value of the ionizable
functional group(s), (2) the solubility of the ionized and unionized forms in
the solution, and (3) the solubilization capacity of the micelles for the ionized
and union-ized forms. Generally, the unionized form is the more hydrophobic
form and is solubilized to a greater extent in the micelles than the ionized
form.
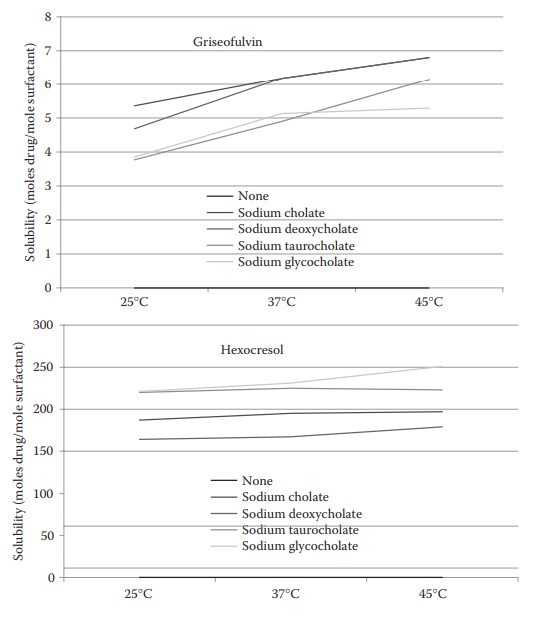
Figure 10.5 Effect of temperature and surfactant type on the micellar
solubilization of griseofulvin and hexocresol. (Modified from Bates, T.R,
Gilbaldi, M. and Kanig, J.I. J. Pharm. Sci., 55, 191, 1966. With Permission.)
2. Pharmaceutical applications
Several
insoluble drugs have been formulated by using micellar solubiliza-tion. For
example:
·
Phenolic compounds, such as cresol, chlorocresol, and
chloroxylenol, are solubilized with soap to form clear solutions for use as
disinfectants.
·
Polysorbates have been used to solubilize steroids in
ophthalmic formulations.
·
Polysorbate are used to prepare aqueous injections of the
water-insoluble vitamins A, D, E, and K.
·
Nonionic surfactants are efficient solubilizers of iodine.
3. Thermodynamics/spontaneity
Micellar
solubilization involves partitioning of the drug between the micel-lar phase
and the aqueous solvent. Thus, the standard free energy of solubi-lization, ∆Gs, can be computed from the
partition coefficient, K, of the drug
between the micelle and the aqueous medium:
∆Gs = −RT In K (10.1)
where:
R is the gas constant
T is the absolute
temperature
Change
in free energy with micellization can be expressed in terms of the change in
enthalpy (∆Hs) and entropy
(∆Ss) as:
∆Gs = ∆Hs − T ∆Ss (10.2)
Thus,
∆H s − T∆ Ss = −RT In K
Or,
In
K = − − ∆Hs/R ⋅ 1/T + constant
where
the constant is ∆Ss/R, assuming that the change in entropy
from micellization is constant. Thus, experimental determination of enthalpy of
micellization can be a useful tool to predict ∆Gs, which, in turn, indicates whether micellar
incorporation of a drug would be spontaneous. When ∆Gs is negative, solubilization process is spontaneous.
When ∆Gs is positive,
solubilization does not occur.
Example 1: Given ∆Hs = 2830 cal/mol and ∆Ss = −26.3 cal/K mol, does
ammonium chloride spontaneously transfer from water to micelles?
∆Gs = ∆Hs − T∆ Ss = 2830 cal/mol − (298K)( − 26.3 cal/kmol)
which
is positive, indicating that micellar solubilization (transfer) would not
occur.
Example 2: Given ∆Hs
= −1700 cal/mol and ∆Ss = 2.1 cal/K mol, does
amobarbital spontaneously transfer from water to a micellar solution (sodium
lauryl sulfate, 0.06 mol/L)?
∆Gs = ∆Hs − T∆ Ss = 1700 cal/mol − (298K)( − 2 .1 cal/kmol) = −2326 cal/mol
which
is negative, indicating that micellar solubilization (transfer) would indeed
spontaneously occur.
Related Topics
