Types of surfactants
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Surfactants and micelles
Surfactants are generally classified according to the nature of the hydro-philic group.
Types of
surfactants
Surfactants are generally classified according to the nature of the hydrophilic group (Table 10.1). The hydrophilic regions can be anionic (neg-atively charged at certain pH values), cationic (positively charged at certain pH values), or nonionic (not charged at all pH values). In addi-tion, some surfactants possess both positively and negatively charged groups. These surfactants can exist in either or both anionic or cationic states, depending on the pH of the solution and the pKa of the ionizable groups on the surfactants. Such surfactants are known as ampholytic compounds.
1. Anionic surfactants
• Sodium stearate
• Sodium dodecyl sulfate (SDS)
• Sodium dodecyl benzene sulfonate
• Sodium cholate
2. Cationic surfactants
• Hexadecyltrimethylammonium bromide
• Dodecyl pyridinium chloride
Nonionic surfactants
• Heptaoxyethylene monohexadecyl ether
3. Ampholytic (Zwitterionic) surfactants
• N-dodecyl alanine
• Lecithin
Table 10.1 Classification of surfactants
1. Anionic surfactants
The
hydrophilic group of anionic surfactants carries a negative charge, such as
R-COO−, RSO4−, or RSO3−,
where R represents an organic group. Anionic surfactants have high
hydrophilicity and are used as detergents and foaming agents, such as in
shampoos. Examples of anionic surfac-tants include soap (sodium salt of fatty
acids, R-COO−Na+), sodium dodecyl sulfate (C12H25SO4Na+)
(SDS), alkylpolyoxyethylene sulfate (R-[CH2CH2O]nSO4−),
and alkylbenzene sulfonate (R-C6H 5-SO3−).
Some of these surfactants, such as SDS,
also known as sodium lauryl sulfate (SLS)
(Figure 10.1), are used to create sink
conditions during in vitro
drug-release studies for new drug product development. It is very water soluble
and has bacteriostatic action against gram-positive bacteria. Therefore, SLS
also finds use as a preoperative skin cleaner and in medicated shampoos.
2. Cationic surfactants
Cationic
surfactants have a cationic group, a functional group that can be positively
charged at certain pH values, as the hydrophilic portion of the molecule. For
example, primary (RNH2), secondary (R2NH), or tertiary
amines (R3N) are positively charged at low pH values. However,
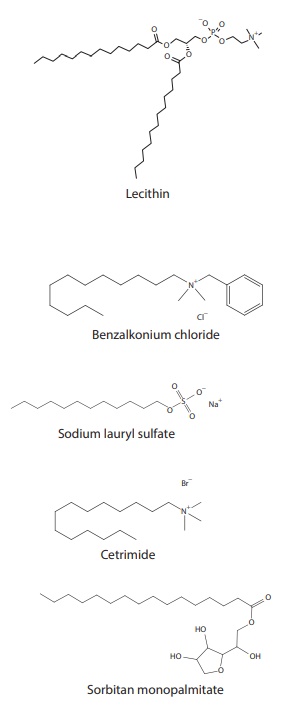
Figure 10.1 Structures of some surfactants.
Most cationic surfactants are
quaternary derivatives of alkylamines, for example, alkyl trimethyl ammonium
salts, dialkyl dimethyl ammonium salts, and alkyl benzyl dimethyl ammonium
salts.
Cationic
surfactants are used in fabric softeners and hair conditioners. In addition,
cationic surfactants can destabilize biological membranes due to the
interaction of their cationic groups with the negatively charged phospholipids
on the cell membranes. This results in their germicidal activ-ity. Thus, the
quaternary ammonium and pyridinium cationic surfactants have bactericidal
activity against a wide range of gram-positive and some gram-negative organisms
and are commonly used as preservatives in phar-maceutical formulations. They
may also be used on the skin for cleans-ing of wounds. For example, solutions
containing 0.1%–1% cetrimide (Figure 10.1) are used for cleaning the skin, wounds,
and burns, as well as for cleaning contaminated vessels. Benzalkonium chloride (Figure 10.1)
is a mixture of alkyl benzyl dimethyl ammonium chlorides. Its dilute solu-tion
may be used for the preoperative disinfection of the skin and mucous membranes,
for application to burns and wounds, and for cleaning poly-ethylene tubing and
catheters. Benzalkonium chloride is also used as a preservative in eye drops.
3. Nonionic surfactants
Nonionic
surfactants contain ether [–(CH2CH2O)nOH]
and/or hydroxyl [–OH] hydrophilic groups. Thus, these surfactants are
nonelectrolytes; that is, their hydrophilic groups do not ionize at any pH
value. Nonionic surfactants are commonly used for stabilizing oil-in-water
(o/w) and water-in-oil (w/o) emulsions. Since the nonionic surfactants do not
contain an ionizable group, their properties are much less sensitive to changes
in the pH of the medium and the presence of electrolytes. In addition, they
have fewer interactions with cell membranes compared with the anionic and
cationic surfactants. Thus, nonionic surfactants are preferred for oral and
parenteral formulations because of their low tissue irritation and toxicity.
Most
commonly used nonionic surfactants include Spans
and Tweens. Sorbitan fatty acid esters (Spans), such as sorbitan
monopalmitate (Figure 10.1), are oil-soluble
emulsifiers that promote the formation of w/o emulsions. Polyethylene glycol
sorbitan fatty acid esters (Tweens) are water-soluble emulsifiers that promote
the formation of o/w emulsions. The Spans and the Tweens come in different
molecular weight or size ranges, which differ in their physical properties.
4. Ampholytic surfactants
Ampholytic
surfactants possess both cationic and anionic groups in the same molecule.
Their ionization state in solution is dependent on the pH of the medium and the
pKa of ionizable groups.
For example, the acidic func-tional groups, such as carboxylate, sulfate, and
sulfonate, are negatively charged (ionized) at pH > pKa, while the basic functional groups, such as amines,
are positively charged (ionized) at pH < pKa. The extent of ioniza-tion of functional groups, that
is, the proportion of molecules in solution that bear the positive or the
negative charge, at a given pH is governed by the Henderson–Hasselbalch
equation, discussed elsewhere in this book. Lecithin
(Figure 10.1), for example, is an
ampholytic surfactant and is used for
parenteral emulsions.
Related Topics
