Review questions answers
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Colloidal dispersions
Pharmaceutical Drugs and Dosage: Colloidal dispersions - Review questions answers
Review questions
9.1 Which of the following
statements about lyophilic colloidal disper-sions is TRUE?
A. They tend to be more
sensitive to the addition of electrolytes than lyophobic systems
B. They tend to be more
viscous than lyophobic systems
C. They can be
precipitated by prolonged dialysis
D. They separate
rapidly
E. All of the above
F. None of the above
9.2 Compounds
that tend to accumulate at interface and reduce surface or interfacial tension are
known as:
A. Antifoaming agents
B. Detergents
C. Wetting agents
D. Surfactants
E. Interfacial agents
9.3 Indicate which of
the following statements is TRUE and which is FALSE:
A. Particle size of
molecular dispersions is larger than a colloidal dispersion.
B. Zeta potential
influences colloidal stability.
C. Nernst potential is
higher than zeta potential.
D. Zeta potential is
electrothermodynamic in nature.
E. Hydrophilic colloids
form turbid solutions.
9.4 Classify disperse
systems based on the particle size of their dispersed phase. Which of these
systems are not visible to the naked eye?
9.5 A. List three
mechanisms involved in acquisition of surface charge in a molecule.
B.
Formulation of amino acids as solutions for parenteral adminis-tration requires
careful consideration of the isoelectric point and the ionization status of the
amino acids. Consider that your labo-ratory is given the amino acid alanine
(structure given below) to be formulated into a solution.
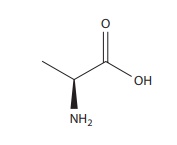
i. Which chemical groups in alanine will affect its
ionization.
ii. Assign either of the two pK values (2.35 and 9.69) of
alanine to each group.
iii. Predict the structure of l-alanine at pH 2, 7, and 10.
9.6 A. What is zeta
potential?
B. Zeta potential of the particles is routinely used for
assessing the stability of pharmaceutical emulsions and suspensions. Suggest a
reason why the surface charge of the particles is not used for this purpose?
9.7 Define and
differentiate aggregation and coagulation in a colloidal system.
9.8 A. Define Stokes’ law.
B. Using the Stokes’ law equation, explain how we can
minimize the sedimentation and creaming phenomena.
C. Sedimentation by ultracentrifugation is often utilized to
deter-mine the particle size of submicron particles. Suggest the principle
behind this application.
D. Suggest two reasons why this method is more suited to
water-insol-uble compounds than to water-soluble molecules.
9.9 A
lyophilic colloid can be:
A. Hydrophilic
B. Hydrophobic
C. Lyophobic
D. All of the above
E. None of the above
Answers:
9.1 B. Most lyophilic
colloids are polymeric molecules including gelatin and acacia; they
spontaneously form colloidal solution, and tend to be viscous. Dispersion of
lyophilic colloids is stable in the pres-ence of electrolytes.
9.2 D. Surfactants
accumulate at the interface and lower the interfacial tension between oil and
water phases.
9.3 A. False
B. True
C. False
D. False
E. False
9.4 Based on their
particle size, colloidal systems are classified into molec-ular dispersions,
colloidal dispersions, and coarse dispersions. Only coarse dispersions are
visible to the naked eye.
9.5 A. Most substances
acquire a surface electric charge when brought into contact with a polar
medium, the possible charging mechanisms being ionization, ion adsorption, and
ion dissolution.
·
Ionization: If the charge
arises from ionization, the charge on the
particles will be the function of pH and pKa.
Proteins acquire their charge mainly through the ionization of carboxyl and
amino groups to give COO− and NH3+ ions. Ionization
depends strongly on pH of the medium. At low pH, a protein molecule will be
positively charged, –NH2 → NH3+, and at
high pH it will be negatively charged, –COOH → COO−. At a certain pH, specific for
each individual protein, the total number of positive charges will be equal to
the total number of negative charges, and the net charge will be zero. This pH
is termed the isoelectric point of the protein.
·
Ion adsorption: A net surface
charge can be acquired by the unequal
adsorption of oppositely charged ions. Surfaces that are already charged have a
tendency to adsorb counterions, which may reverse the surface charge.
·
Ion dissolution: Ionic substances
can acquire a surface charge by
virtue of unequal dissolution of the oppositely charged ions of which they are
composed. For example, the particle of silver iodide in a solution with excess
[I−] will carry a negative charge, but the charge will be positive if excess
[Ag+] is present.
B. i. –COOH and NH2
ii. –COOH has 2.35 and NH2 has 9.69
iii.
Low pH, NH3+; median pH 7, both groups ionized; basic pH, COO−
9.6 A. Zeta potential is
defined as the difference in potential between the surface of the tightly bound
layer of solvent/shear plane and the electroneutral region of the solution.
B. Electrophoretic properties are affected by the net charge
on a particle, which includes that of an immobile solvent layer.
9.7 A. When the particles
adhere by stronger forces, the phenomenon is called aggregation. Because of the large surface free energy of the
dispersed-phase particles in emulsions, they tend to associate together by weak
van der Waals forces forming light, fluffy con-glomerates. This phenomenon is
called flocculation. Coagulation is
the condition when the dispersed-phase particles merge with each other to form
a single phase.
B. Coagulation is an irreversible process and leads to
caking, whereas flocculation is the process of forming light fluffy
conglomerates, which are reversible on shaking.
9.8 A. Stokes’ law defines
the velocity of sedimentation as a function of the viscosity of the medium and
the radius and the density of particles as per the following equation:
V = D2(ρ − ρ0)g / 18η′0
B.
Creaming is the upward movement of dispersed droplets relative to the
continuous phase, whereas sedimentation, the reverse pro-cess, is the downward
movement of particles. These processes take place because of the density
differences in the two phases and can be reversed by shaking. However, creaming
is undesir-able because it provides the possibility of inaccurate dosing and
increases the likelihood of some coalescence, which may take place owing to the
close proximity of the globules in the cream.
Factors
that influence the rate of creaming are similar to those involved in the
sedimentation rate as indicated by Stokes’ law:
v= d2(ρs − ρ0)g / 18η0
where:
v is the velocity of
creaming
d is the globule
diameter
ρs and ρ0 are the densities of disperse phase
and dispersion medium
ή0
is the viscosity of the dispersion medium (poise)
g is the acceleration
of gravity (981 cm/s2)
C. According to Stokes’ equation, we can minimize the rate
of creaming and sedimentation by (i) reducing the globule size, (ii) decreasing
the density difference between the two phases, and (iii) increasing the
viscosity of the continuous phase. This may be achieved by homogenizing the
emulsion to reduce the globule size and increasing the viscosity of the
continuous phase by the use of thickening agents such as tragacanth or
methylcellulose for o/w emulsions and soft paraffin for w/o emulsions.
D. The rate of sedimentation is directly proportional to the
diameter of particles if density/shape is the same.
E. Water-soluble compounds will dissolve while being
processed, causing increase in viscosity of the medium and reduction in
diameter. According to Stokes’ law, viscosity increase will affect the results.
9.9 D.
Related Topics
