Surfactants
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Surfactants and micelles
A surfactant molecule has two distinct regions—hydrophilic (water-liking) and hydrophobic (water-hating).
Surfactants
A surfactant molecule has two distinct regions—hydrophilic (water-liking) and hydrophobic (water-hating). The existence of such two
regions in a molecule is known as amphipathy,
and the molecules are consequently referred to as amphipathic molecules or amphiphiles.
The hydrophilic por-tions are typically the functional groups that bear
electronegative atoms that can form hydrogen bonds with water and can
participate in dipole– dipole interactions. The hydrophobic portions are
usually saturated or unsaturated hydrocarbon chains or, less commonly, a
heterocyclic or aro-matic ring system. Depending on the number and nature of
the polar and nonpolar functional groups present, the amphiphile may be
predominantly hydrophilic, predominantly lipophilic, or almost equal in
hydrophilic and lipophilic characters. For example, straight-chain alcohols,
amines, and acids are amphiphiles that change from being predominantly
hydrophilic to predominantly lipophilic as the number of carbon atoms in the
alkyl chain is increased.
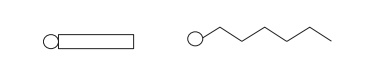
Surfactants
are usually depicted with a circle representing a polar (hydro-philic) head
group and a wiggly chain or a rectangular box depicting a nonpolar (lipophilic)
region.
The
surface activity (ability to reduce surface/interfacial tension) of a
surfactant depends on its ability to preferentially partition into the
inter-face, which, in turn, depends on the balance between its hydrophilic and
hydrophobic properties. The surfactant molecules localize at the surface, with
the hydrophobic regions pointing toward and bonding the hydro-phobic liquid (or
air), while the hydrophilic regions pointing toward and bonding the aqueous or
hydrophilic liquid. Thus, the surfactant mol-ecules replace the bulk liquid
molecules on the surface with molecules that show mutual attraction for both
sides of the surface, which reduces surface tension. For air–water surfaces, an
increase in the length of the hydrocarbon chain of a surfactant results in an
increased surface activ-ity. Conversely, an increase in the hydrophilicity
results in a decreased surface activity.
Related Topics
