Types of colloidal systems
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Colloidal dispersions
On the basis of the type and extent of molecular interactions of the dis-persed phase with the dispersion medium, colloidal systems can be classi-fied into three groups: lyophilic, lyophobic, and association colloids.
Types of colloidal
systems
On
the basis of the type and extent of molecular interactions of the dispersed
phase with the dispersion medium, colloidal systems can be classi-fied into
three groups: lyophilic, lyophobic, and association colloids.
1. Lyophilic colloids
A
lyophilic colloid (solvent loving) is a system in which the
dispersed phase has an affinity for the dispersion medium. Depending on the
type of disper-sion medium (solvent), both lipophilic (i.e., lipid-loving,
which represents the same characteristics as hydrophobic or water-hating) and
hydrophilic (i.e., water-loving, which represents the same characteristics as
lipophobic or lipid-hating) colloids can be lyophilic (solvent-loving). Thus, lipophilic colloids are a dispersion of the lipophilic or hydrophobic material
in an organic solvent. Hydrophilic colloids are a dispersion of
hydrophilic mate-rial in an aqueous medium.
Owing
to their affinity for the dispersion medium, lyophilic materials form colloidal
dispersions with relative ease. Examples of lyophilic colloids include gelatin,
acacia, proteins (such as insulin), nucleic acids, albumin, rubber, and
polystyrene. Of these, the first five produce lyophilic colloids in aqueous
dispersion medium and are called hydrophilic colloids. Rubber and polystyrene
form lyophilic colloids in organic solvents and are thus referred to as
lipophilic colloids.
2. Lyophobic colloids
Lyophobic
(solvent-hating) colloids are
composed of materials that have little attraction, if any, for the dispersion
medium. Lyophobic colloids are intrinsically physically unstable. These are
formed by the mismatch of pre-ferred molecular interactions of the dispersed
phase and the dispersion medium. For example, water and hydrophilic molecules
prefer stronger hydrogen bonding, dipole–dipole interactions, and electrostatic
interac-tions, while lipids and hydrophobic molecules prefer weaker van der
Waals interactions. Examples of lyophobic colloids are gold, silver, arsenous
sul-fate, and silver iodide. Thus, dispersion of hydrophobic molecules,
par-ticles, or material in an aqueous medium results in hydrophobic colloids. Special methods and energy input are required
to prepare stable lyophobic colloids, as they do not form spontaneously.
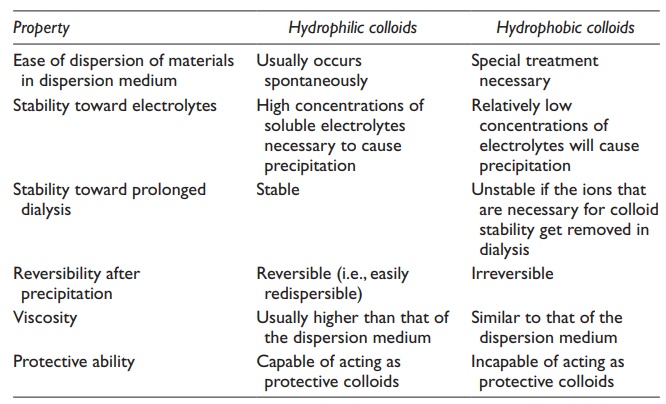
Differences
in the properties of hydrophilic and hydrophobic colloids are summarized in Table 9.1.
3. Association colloids
Association, or amphiphilic colloids are formed by the
grouping or self-association of the dispersed phase, which is amphiphilic
(e.g., surface-active agents). These molecules exhibit both lyophilic and
lyophobic properties. At low concentrations, amphiphiles exist separately as
molecular dispersions or true solutions and do not form a colloid. However, at
higher concentra-tions, self-association of several monomers, or individual
molecules occurs
Table 9.1 Differences in properties of hydrophilic and hydrophobic colloids
The number of monomers that aggregate to form a micelle is called the aggregation number. As with lyophilic
colloids, formation of association colloids is spontaneous, provided that the
concentration of the amphiphile in solution exceeds the CMC.
Related Topics
