One-Compartment Open Model: Intravenous Infusion
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Compartment Modelling
Rapid i.v. injection is unsuitable when the drug has potential to precipitate toxicity or when maintenance of a stable concentration or amount of drug in the body is desired.
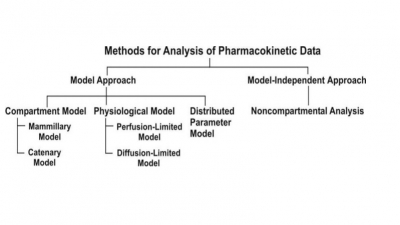
One-Compartment Open Model
Intravenous Infusion
Rapid i.v. injection is unsuitable when the drug
has potential to precipitate toxicity or when maintenance of a stable
concentration or amount of drug in the body is desired. In such a situation,
the drug (for example, several antibiotics, theophylline, procainamide, etc.)
is administered at a constant rate (zero-order) by i.v. infusion. In contrast
to the short duration of infusion of an i.v. bolus (few seconds), the duration
of constant rate infusion is usually much longer than the half-life of the
drug.
Advantages of zero-order infusion of drugs
include—
1. Ease of control of rate of
infusion to fit individual patient needs.
2. Prevents fluctuating maxima
and minima (peak and valley) plasma level, desired especially when the drug has
a narrow therapeutic index.
3. Other drugs, electrolytes and
nutrients can be conveniently administered simultaneously by the same infusion
line in critically ill patients.
The model can be represented as follows:

At any time during infusion, the rate of change in
the amount of drug in the body, dX/dt is the difference between the zero-order
rate of drug infusion Ro and first-order rate of elimination, –KEX:
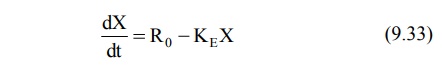
Integration and rearrangement of above equation
yields:
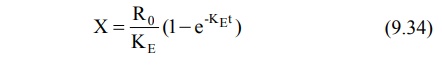
Since X = Vd C, the equation 9.34 can be
transformed into concentration terms as follows:
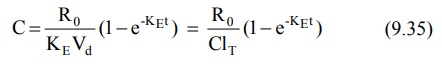
At the start of constant rate infusion, the amount
of drug in the body is zero and hence, there is no elimination. As time passes,
the amount of drug in the body rises gradually (elimination rate less than the
rate of infusion) until a point after which the rate of elimination equals the
rate of infusion i.e. the concentration of drug in plasma approaches a constant
value called as steady-state, plateau or infusion equilibrium (Fig. 9.3.).
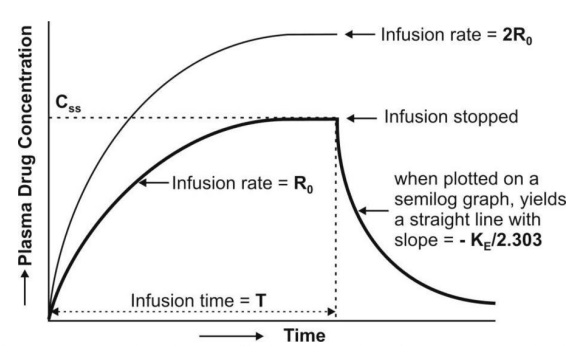
Fig. 9.3. Plasma concentration-time profile
for a drug given by constant rate i.v. infusion (the two curves indicate different infusion rates Ro and 2Ro
for the same drug)
At steady-state, the rate of change of amount of
drug in the body is zero, hence, the equation 9.33 becomes:
Zero = R 0 - K E Xss
or KE Xss = Ro (9.36)
Transforming to concentration terms and rearranging
the equation:

where, Xss and Css are amount
of drug in the body and concentration of drug in plasma at steady-state
respectively. The value of KE (and hence t½) can be
obtained from the slope of straight line obtained after a semilogarithmic plot (log
C versus t) of the plasma concentration-time data gathered from the time when
infusion is stopped (Fig. 9.3). Alternatively, KE can be calculated
from the data collected during infusion to steady-state as follows:
Substituting Ro/ClT = Css
from equation 9.37 in equation 9.35 we get:
C = C ss (1- e-KEt ) (9.38)
Rearrangement yields:
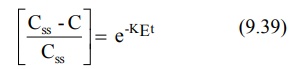
Transforming into log form, the equation becomes:
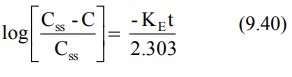
A semilog plot of (Css – C)/Css
versus t results in a straight line with slope –KE/2.303 (Fig. 9.4).
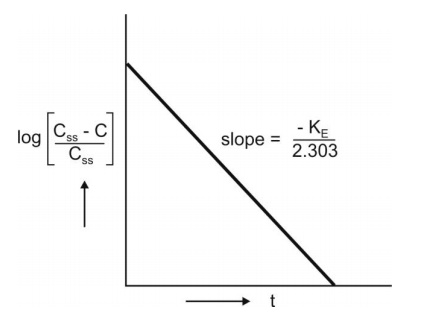
Fig. 9.4 Semilog plot to compute KE from infusion data upto steady-state
The time to reach steady-state concentration is
dependent upon the elimination half-life and not infusion rate. An increase in
infusion rate will merely increase the plasma concentration attained at
steady-state (Fig. 9.3). If n is the number of half-lives passed since the
start of infusion (t/t½), equation 9.38 can be written as:
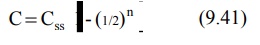
The percent of Css achieved at the end
of each t½ is the sum of Css at previous t½
and the concentration of drug remaining after a given t½ (Table
9.3).
TABLE 9.3
Percent of Css attained at the end of a given t½
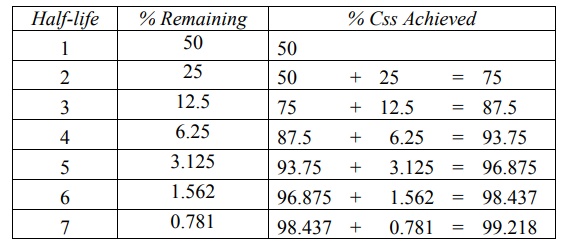
For therapeutic purpose, more than 90% of the
steady-state drug concentration in the blood is desired which is reached in 3.3
half-lives. It takes 6.6 half-lives for the concentration to reach 99% of the
steady-state. Thus, the shorter the half-life (e.g. penicillin G, 30 min),
sooner is the steady-state reached.
Infusion Plus Loading Dose: It takes
a very long time for the drugs having longer half-lives before the plateau
concentration is reached (e.g. phenobarbital, 5 days). Thus, initially, such
drugs have subtherapeutic concentrations. This can be overcome by administering
an i.v. loading dose large enough to
yield the desired steady-state immediately upon injection prior to starting the
infusion. It should then be followed immediately by i.v. infusion at a rate
enough to maintain this concentration (Fig. 9.5).
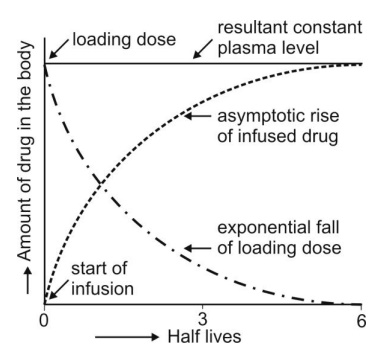
Fig. 9.5 Intravenous infusion with loading dose. As the amount of bolus dose
remaining in the body falls, there
is a complementary rise resulting from the infusion
Recalling once again the relationship X = VdC,
the equation for computing the loading dose Xo,L can be given:
X0,LCss Vd (9.42)
Substitution of Css = Ro/KEVd
from equation 9.37 in above equation yields another expression for loading dose
in terms of infusion rate:

The equation describing the plasma
concentration-time profile following simultaneous i.v. loading dose (i.v.
bolus) and constant rate i.v. infusion is the sum of two equations describing
each process (i.e. modified equation 9.5 and 9.35):
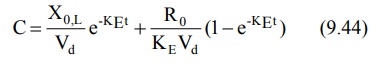
If we substitute CssVd for Xo,L
(from equation 9.42) and CssKEVd for Ro
(from equation 9.37) in above equation and simplify it, it reduces to C = Css
indicating that the concentration of drug in plasma remains constant (steady)
throughout the infusion time.
Assessment of Pharmacokinetic Parameters
The first-order elimination rate constant and
elimination half-life can be computed from a semilog plot of post-infusion
concentration-time data. Equation 9.40 can also be used for the same purpose.
Apparent volume of distribution and total systemic clearance can be estimated
from steady-state concentration and infusion rate (equation 9.37). These two
parameters can also be computed from the total area under the curve (Fig. 9.3) till
the end of infusion:
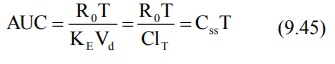
where, T = infusion time.
The above equation is a general expression which
can be applied to several pharmacokinetic models.
Related Topics