Steroid Hormones
| Home | | Biochemistry |Chapter: Biochemistry : Cholesterol, Lipoprotein, and Steroid Metabolism
Cholesterol is the precursor of all classes of steroid hormones: glucocorticoids (for example, cortisol), mineralocorticoids (for example, aldosterone), and the sex hormones (that is, androgens, estrogens, and progestins).
STEROID HORMONES
Cholesterol is the
precursor of all classes of steroid hormones: glucocorticoids (for example,
cortisol), mineralocorticoids (for example, aldosterone), and the sex hormones
(that is, androgens, estrogens, and progestins) as shown in Figure 18.24).
[Note: Glucocorticoids and mineralocorticoids are collectively called
corticosteroids.] Synthesis and secretion occur in the adrenal cortex
(cortisol, aldosterone, and androgens), ovaries and placenta (estrogens and
progestins), and testes (testosterone). Steroid hormones are transported by the
blood from their sites of synthesis to their target organs. Because of their
hydrophobicity, they must be complexed with a plasma protein. Plasma albumin
can act as a nonspecific carrier and does carry aldosterone. However, specific
steroid-carrier plasma proteins bind the steroid hormones more tightly than
does albumin (for example, corticosteroid-binding globulin, or transcortin, is
responsible for transporting cortisol). A number of genetic diseases are caused
by deficiencies in specific steps in the biosynthesis of steroid hormones. Some
representative diseases are described in Figure 18.25.
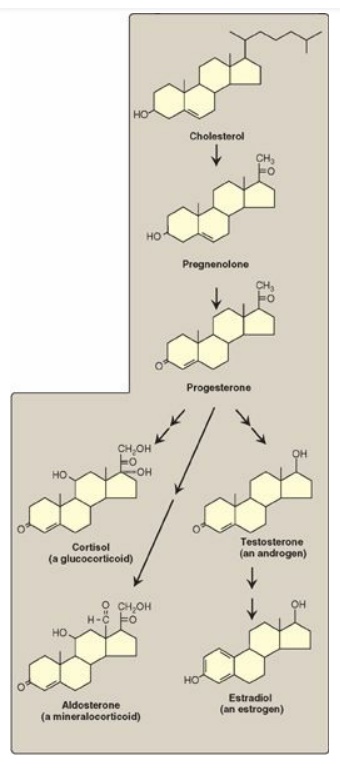
Figure 18.24 Key steroid hormones.
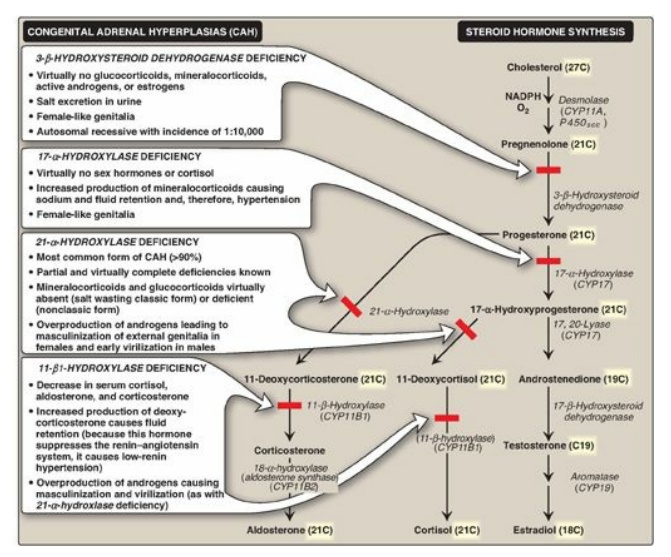
Figure 18.25 Steroid hormone synthesis and associated diseases. [Note: 3-β-Hydroxysteroid dehydrogenase, CYP17, and CYP11B2 are bifunctional enzymes. Synthesis of testosterone and the estrogens from cholesterol occurs primarily outside of the adrenal gland.] NADPH = nicotinamide adenine dinucleotide phosphate; CYP = cytochrome P450.
A. Synthesis of steroid hormones
Synthesis involves shortening the hydrocarbon chain of cholesterol and hydroxylation of the steroid nucleus. The initial and rate-limiting reaction converts cholesterol to the 21-carbon pregnenolone. It is catalyzed by the cholesterol side-chain cleavage enzyme (desmolase, P450scc), a cytochrome P450 (CYP) mixed-function oxidase of the inner mitochondrial membrane. NADPH and O2 are required for the reaction. The cholesterol substrate can be newly synthesized, taken up from lipoproteins, or released by an esterase from cholesteryl esters stored in the cytosol of steroidogenic tissues. The cholesterol moves to the outer mitochondrial membrane. An important control point is the subsequent movement from the outer to the inner mitochondrial membrane. This process is mediated by StAR (steroidogenic acute regulatory) protein. Pregnenolone is the parent compound for all steroid hormones (see Figure 18.25). It is oxidized and then isomerized to progesterone, which is further modified to the other steroid hormones by hydroxylation reactions that occur in the ER and mitochondria. Like desmolase, the enzymes primarily are CYP proteins. A defect in the activity or amount of an enzyme in this pathway can lead to a deficiency in the synthesis of hormones beyond the affected step and to an excess in the hormones or metabolites before that step. Because all members of the pathway have potent biologic activity, serious metabolic imbalances occur with enzyme deficiencies (see Figure 18.25). Collectively these disorders are known as the congenital adrenal hyperplasias. [Note: Addison disease, due to autoimmune destruction of the adrenal cortex, is characterized by adrenocortical insufficiency.]
B. Secretion of adrenal cortical steroid hormones
Steroid hormones are
secreted on demand from their tissues of origin in response to hormonal
signals. The corticosteroids and androgens are made in different regions of the
adrenal cortex and are secreted into blood in response to different signals.
1. Cortisol: Its production in the middle layer (zona
fasciculata) of the adrenal cortex is controlled by the hypothalamus, to which
the pituitary gland is attached (Figure 18.26). In response to severe stress
(for example, infection), corticotropin-releasing hormone (CRH), produced by
the hypothalamus, travels through capillaries to the anterior lobe of the
pituitary, where it induces the production and secretion of adrenocorticotropic
hormone (ACTH). The polypeptide ACTH, the “stress hormone,” stimulates the
adrenal cortex to synthesize and secrete the glucocorticoid cortisol. Cortisol
allows the body to respond to stress through its effects on intermediary
metabolism (for example, increased gluconeogenesis) and the inflammatory and
immune responses. As cortisol levels rise, the release of CRH and ACTH is
inhibited. [Note: ACTH binds to a membrane G protein–coupled receptor,
resulting in cyclic AMP (cAMP) production and activation of protein kinase A
(PKA). PKA phosphorylates and activates both the esterase that converts
cholesteryl ester to free cholesterol and StAR protein.]
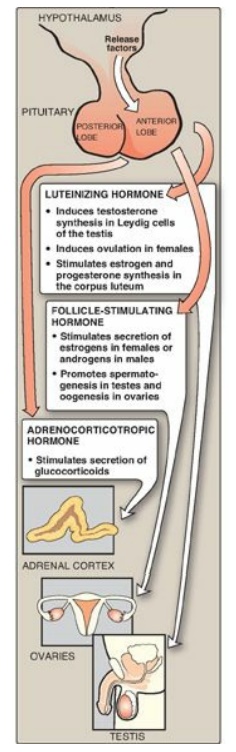
Figure 18.26 Pituitary hormone stimulation of steroid hormone synthesis and secretion.
2. Aldosterone: Its production in the outer layer (zona glomerulosa) of the adrenal cortex is induced by a decrease in the plasma Na+/K+ ratio, and by the hormone angiotensin II. Angiotensin II (an octapeptide) is produced from angiotensin I (a decapeptide) by angiotensin-converting enzyme (ACE), an enzyme found predominantly in the lungs but also distributed widely in the body. [Note: Angiotensin I is produced in the blood by cleavage of an inactive precursor, angiotensinogen, secreted by the liver. Cleavage is accomplished by the enzyme renin, made and secreted by the kidney.] Angiotensin II binds to cell-surface receptors. However, in contrast to ACTH, its effects are mediated through the phosphatidylinositol 4,5-bisphosphate pathway and not by cAMP. Aldosterone’s primary effect is on the kidney tubules, where it stimulates sodium and water uptake and potassium excretion (Figure 18.27). [Note: An effect of aldosterone is an increase in blood pressure. Competitive inhibitors of ACE are used to treat renin-dependent hypertension.]
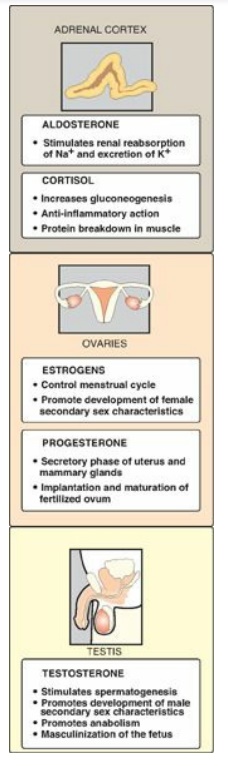
Figure 18.27 Actions of steroid hormones.
3. Androgens: Both the inner (zona reticularis) and middle
layers of the adrenal cortex produce androgens, primarily
dehydroepiandrosterone and androstenedione. Although adrenal androgens
themselves are weak, they are converted in peripheral tissues to testosterone,
a stronger androgen, and to estrogens.
Estrogens are derived from androstenedione and
testosterone by aromatase (CYP19) . Aromatase inhibitors are used in the
treatment of estrogen-responsive breast cancer in postmenopausal women.
C. Secretion of steroid hormones from gonads
The testes and ovaries
synthesize hormones necessary for sexual differentiation and reproduction. A
single hypothalamic-releasing factor, gonadotropin-releasing hormone,
stimulates the anterior pituitary to release the glycoproteins luteinizing
hormone (LH) and follicle-stimulating hormone (FSH). Like ACTH, LH and FSH bind
to surface receptors and cause an increase in cAMP. LH stimulates the testes to
produce testosterone and the ovaries to produce estrogens and progesterone (see
Figure 18.27). FSH regulates the growth of ovarian follicles and stimulates
testicular spermatogenesis.
D. Mechanism of steroid hormone action
Each steroid hormone diffuses across the plasma membrane of its target cell and binds to a specific cytosolic or nuclear receptor. These receptor–ligand complexes accumulate in the nucleus, dimerize, and bind to specific regulatory DNA sequences (hormone-response elements [HREs]) in association with coactivator proteins, thereby causing promoter activation and increased transcription of targeted genes (Figure 18.28). An HRE is found in the promoter or an enhancer element for genes that respond to a specific steroid hormone, thus insuring coordinated regulation of these genes. Hormone–receptor complexes can also inhibit transcription in association with corepressors. [Note: The binding of a hormone to its receptor causes a conformational change in the receptor that uncovers its DNA-binding domain, allowing the complex to interact through a zinc-finger motif with the appropriate sequence on the DNA. Receptors for the steroid hormones, plus those for thyroid hormone, retinoic acid, and 1,25-dihydroxycholecalciferol (vitamin D), are members of a “superfamily” of structurally related gene regulators that function in a similar way.]
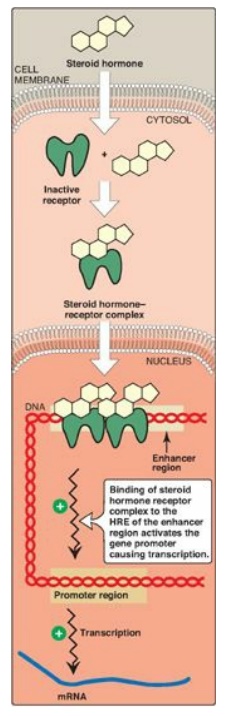
Figure 18.28 Activation of
transcription by interaction of steroid hormone-receptor complex with hormone
response element (HRE). The receptor contains domains that bind the hormone,
DNA, and proteins that relax the DNA. mRNA = messenger RNA.
E. Further metabolism of steroid hormones
Steroid hormones are
generally converted into inactive metabolic excretion products in the liver.
Reactions include reduction of unsaturated bonds and the introduction of
additional hydroxyl groups. The resulting structures are made more soluble by
conjugation with glucuronic acid or sulfate (from 3I -phosphoadenosyl-5I-
phosphosulfate). Approximately 20%–30% of these metabolites are secreted into
the bile and then excreted in the feces, whereas the remainder are released
into the blood and filtered from the plasma in the kidney, passing into the
urine. These conjugated metabolites are fairly water soluble and do not need
protein carriers.
Related Topics
