Digestion of Dietary Proteins
| Home | | Biochemistry |Chapter: Biochemistry : Amino Acids: Disposal of Nitrogen
Most of the nitrogen in the diet is consumed in the form of protein, typically amounting to 70–100 g/day in the American diet.
DIGESTION OF DIETARY PROTEINS
Most of the nitrogen in
the diet is consumed in the form of protein, typically amounting to 70–100
g/day in the American diet (see Figure 19.2). Proteins are generally too large
to be absorbed by the intestine. [Note: An example of an exception to this rule
is that newborns can take up maternal antibodies in breast milk.] They must,
therefore, be hydrolyzed to yield di- and tripeptides as well as individual
amino acids, which can be absorbed. Proteolytic enzymes responsible for
degrading proteins are produced by three different organs: the stomach, the
pancreas, and the small intestine (Figure 19.4).
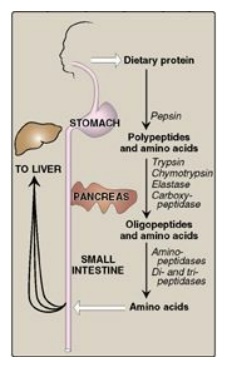
Figure 19.4 Digestion of dietary proteins by the proteolytic enzymes of the gastrointestinal tract.
A. Digestion by gastric secretion
The digestion of
proteins begins in the stomach, which secretes gastric juice, a unique solution
containing hydrochloric acid and the proenzyme pepsinogen.
1. Hydrochloric acid: Stomach acid is too dilute (pH
2–3) to hydrolyze proteins. The acid, secreted by the parietal cells of the
stomach, functions instead to kill some bacteria and to denature proteins,
thereby making them more susceptible to subsequent hydrolysis by proteases.
2. Pepsin: This acid-stable endopeptidase is secreted by the chief cells of the stomach as an inactive zymogen (or proenzyme), pepsinogen. [Note: In general, zymogens contain extra amino acids in their sequences that prevent them from being catalytically active. Removal of these amino acids permits the proper folding required for an active enzyme.] Pepsinogen is activated to pepsin, either by hydrochloric acid or autocatalytically by pepsin molecules that have already been activated. Pepsin releases peptides and a few free amino acids from dietary proteins.
B. Digestion by pancreatic enzymes
On entering the small
intestine, large polypeptides produced in the stomach by the action of pepsin
are further cleaved to oligopeptides and amino acids by a group of pancreatic
proteases that include both endopeptidases (cleave within) and exopeptidases
(cut at an end). [Note: Bicarbonate (HCO3–), also
secreted by the pancreas, raises the pH.]
1. Specificity: Each of these enzymes has a different specificity
for the amino acid R-groups adjacent to the susceptible peptide bond (Figure
19.5). For example, trypsin cleaves only when the carbonyl group of the peptide
bond is contributed by arginine or lysine. These enzymes, like pepsin described
above, are synthesized and secreted as inactive zymogens.
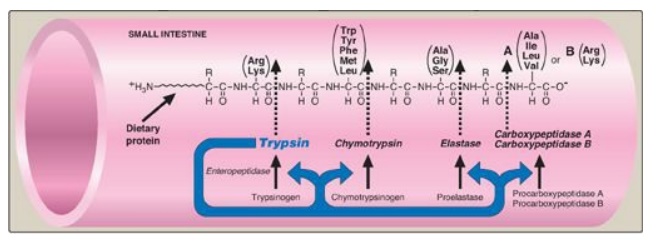
Figure 19.5 Cleavage of dietary protein in the small intestine by pancreatic proteases. The peptide bonds susceptible to hydrolysis are shown for each of the five major pancreatic proteases. [Note: The first three are serine endopeptidases, whereas the last two are exopeptidases. Each is produced from an inactive zymogen.]
2. Release of zymogens: The release and activation of the
pancreatic zymogens is mediated by the secretion of cholecystokinin and
secretin, two polypeptide hormones of the digestive tract.
3. Activation of zymogens: Enteropeptidase (formerly called
enterokinase), an enzyme synthesized by and present on the luminal surface of
intestinal mucosal cells of the brush border membrane, converts the pancreatic
zymogen trypsinogen to trypsin by removal of a hexapeptide from the N-terminus
of trypsinogen. Trypsin subsequently converts other trypsinogen molecules to
trypsin by cleaving a limited number of specific peptide bonds in the zymogen.
Enteropeptidase, thus, unleashes a cascade of proteolytic activity because
trypsin is the common activator of all the pancreatic zymogens (see Figure
19.5).
4. Abnormalities in protein digestion: In individuals with a deficiency in pancreatic secretion (for example, due to chronic pancreatitis, cystic fibrosis, or surgical removal of the pancreas), the digestion and absorption of fat and protein are incomplete. This results in the abnormal appearance of lipids in the feces (a condition called steatorrhea;) as well as undigested protein.
Celiac disease (celiac sprue) is a disease of
malabsorption resulting from immune-mediated damage to the small intestine in
response to ingestion of gluten (or gliadin produced from gluten), a protein
found in wheat, barley and rye.
C. Digestion of oligopeptides by enzymes of the small intestine
The luminal surface of
the intestine contains aminopeptidase, an exopeptidase that repeatedly cleaves
the N-terminal residue from oligopeptides to produce even smaller peptides and
free amino acids.
D. Absorption of amino acids and small peptides
Free amino acids are taken into the enterocytes by a sodium-linked secondary transport system of the apical membrane. Di- and tripeptides, however, are taken up by a proton-linked transport system. The peptides are hydrolyzed in the cytosol to amino acids that are released into the portal system by facilitated diffusion. Therefore, only free amino acids are found in the portal vein after a meal containing protein. These amino acids are either metabolized by the liver or released into the general circulation. [Note: Branched-chain amino acids are important examples of amino acids that are not metabolized by the liver but, instead, are sent from the liver primarily to muscle via the blood.]
Related Topics
