Structure of Glycosphingolipids
| Home | | Biochemistry |Chapter: Biochemistry : Phospholipid, Glycosphingolipid, and Eicosanoid Metabolism
The glycosphingolipids differ from sphingomyelin in that they do not contain phosphate, and the polar head function is provided by a monosaccharide or oligosaccharide attached directly to the ceramide by an O-glycosidic bond.
STRUCTURE OF GLYCOSPHINGOLIPIDS
The glycosphingolipids
differ from sphingomyelin in that they do not contain phosphate, and the polar
head function is provided by a monosaccharide or oligosaccharide attached
directly to the ceramide by an O-glycosidic bond (Figure 17.14 ). The number
and type of carbohydrate moieties present determine the type of glycosphingolipid.
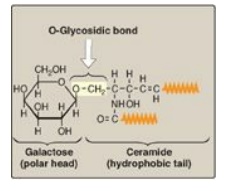
Figure 17.14 Structure of a neutral glycosphingolipid, galactocerebroside. (  is a hydrophobic hydrocarbon chain.)
is a hydrophobic hydrocarbon chain.)
A. Neutral glycosphingolipids
The simplest neutral
(uncharged) glycosphingolipids are the cerebrosides. These are ceramide
monosaccharides that contain either a molecule of galactose (forming
ceramide-galactose or galactocerebroside, the most common cerebroside found in
myelin, as shown in Figure 17.14 ) or glucose (forming ceramide-glucose or
glucocerebroside, which serves primarily as an intermediate in the synthesis
and degradation of the more complex glycosphingolipids). [Note: Members of a group
of galacto- or glucocerebrosides may also differ from each other in the type of
fatty acid attached to the sphingosine.] As their name implies, cerebrosides
are found predominantly in the brain and peripheral nervous tissue, with high
concentrations in the myelin sheath. Ceramide oligosaccharides (or globosides)
are produced by attaching additional monosaccharides to a glucocerebroside, for
example, ceramide-glucose-galactose (also known as lactosylceramide). The
additional monosaccharides can include substituted sugars such as
N-acetylgalactosamine.
B. Acidic glycosphingolipids
Acidic
glycosphingolipids are negatively charged at physiologic pH. The negative
charge is provided by N-acetylneuraminic acid ([NANA], a sialic acid, as shown
in Figure 17.15) in gangliosides, or by sulfate groups in sulfatides.
1. Gangliosides: These are the most complex glycosphingolipids and
are found primarily in the ganglion cells of the CNS, particularly at the nerve
endings. They are derivatives of ceramide oligosaccharides and contain one or
more molecules of NANA. The notation for these compounds is G (for ganglioside)
plus a subscript M, D, T, or Q to indicate whether there is one (mono), two
(di), three (tri), or four (quatro) molecules of NANA in the ganglioside, respectively.
Additional numbers and letters in the subscript designate the monomeric
sequence of the carbohydrate attached to the ceramide. (See Figure 17.15 for
the structure of GM2.) Gangliosides are of medical interest because several
lipid storage disorders involve the accumulation of NANA-containing
glycosphingolipids in cells (see Figure 17.20).
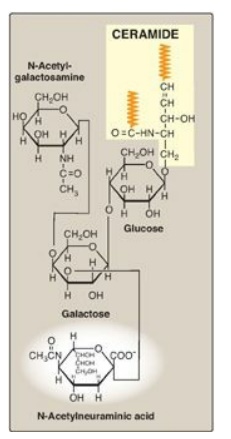
Figure 17.15 Structure of the
ganglioside GM2. ( is a hydrophobic hydrocarbon chain.)
is a hydrophobic hydrocarbon chain.)
2. Sulfatides: These sulfoglycosphingolipids are sulfated
galactocerebrosides that are negatively charged at physiologic pH. Sulfatides
are found predominantly in the brain and kidneys.
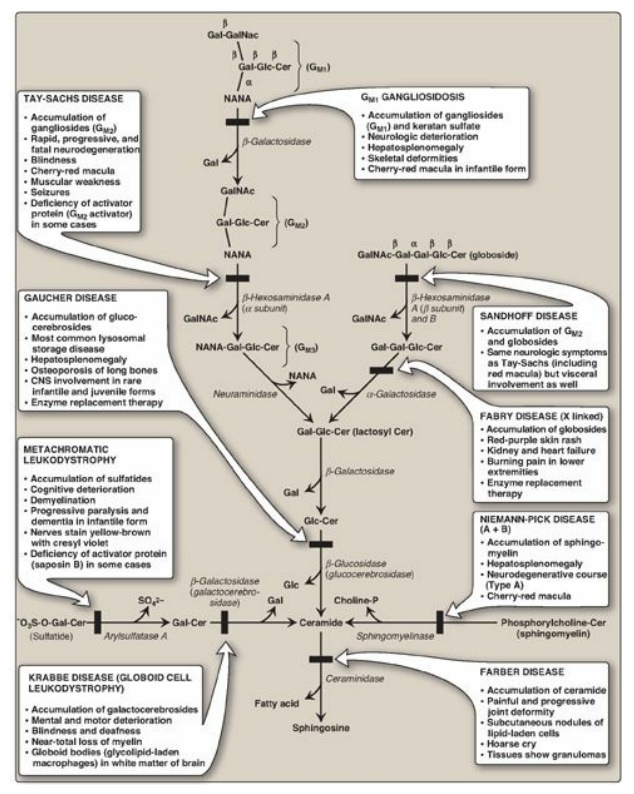
Figure 17.20 Degradation of sphingolipids showing the lysosomal enzymes affected in related genetic diseases, the sphingolipidoses. All of the diseases are autosomal recessive except Fabry disease, which is X linked, and all can be fatal in early life. Cer = ceramide; Gal = galactose; Glc = glucose; GalNAc = N-acetylgalactosamine; NANA = N-acetylneuraminic acid; CNS = central nervous system. SO42- = sulfate.
Related Topics
