Degradation of Phospholipids
| Home | | Biochemistry |Chapter: Biochemistry : Phospholipid, Glycosphingolipid, and Eicosanoid Metabolism
The degradation of phosphoglycerides is performed by phospholipases found in all tissues and pancreatic juice (for a discussion of phospholipid digestion).
DEGRADATION OF PHOSPHOLIPIDS
The degradation of
phosphoglycerides is performed by phospholipases found in all tissues and
pancreatic juice (for a discussion of phospholipid digestion). A number of
toxins and venoms have phospholipase activity, and several pathogenic bacteria
produce phospholipases that dissolve cell membranes and allow the spread of
infection. Sphingomyelin is degraded by the lysosomal phospholipase,
sphingomyelinase (see below).
A. Phosphoglycerides
Phospholipases
hydrolyze the phosphodiester bonds of phosphoglycerides, with each enzyme
cleaving the phospholipid at a specific site. The major phospholipases are
shown in Figure 17.11 . [Note: Removal of the fatty acid from carbon 1 or 2 of
a phosphoglyceride produces a lysophosphoglyceride, which is the substrate for
lysophospholipases. ] Phospholipases release molecules that can serve as second
messengers (for example, DAG and IP3) or that are the substrates for
synthesis of messengers (for example, arachidonic acid). Phospholipases are
responsible not only for degrading phospholipids, but also for “remodeling”
them. For example, phospholipases A1 and A2 remove
specific fatty acids from membrane-bound phospholipids, which can be replaced
with different fatty acids using fatty acyl CoA transferase. This mechanism is
used as one way to create the unique lung surfactant DPCC and to insure that
carbon 2 of PI (and sometimes of PC) is bound to arachidonic acid. [Note: Barth
syndrome, a rare X-linked disorder characterized by cardiomyopathy, muscle
weakness, and neutropenia, is the result of defects in cardiolipin remodeling.]

Figure 17.11 Degradation of
glycerophospholipids by phospholipases. PIP2 = phosphatidylinositol
4,5-bisphosphate; R1 and R2 = fatty acids; X = an
alcohol.
B. Sphingomyelin
Sphingomyelin is
degraded by sphingomyelinase, a lysosomal enzyme that hydrolytically removes
phosphorylcholine, leaving a ceramide. The ceramide is, in turn, cleaved by
ceramidase into sphingosine and a free fatty acid (Figure 17.12 ). [Note: The
ceramide and sphingosine released regulate signal transduction pathways, in
part by influencing the activity of protein kinase C and, thus, the
phosphorylation of its protein substrates. They also promote apoptosis.] Niemann-Pick
disease (Types A and B is an autosomal-recessive disease caused by the
inability to degrade sphingomyelin due to a deficiency of sphingomyelinase, a
type of phospholipase C. In the severe infantile form (Type A, which shows less
than 1% of normal enzymic activity), the liver and spleen are the primary sites
of lipid deposits and are, therefore, greatly enlarged. The lipid consists
primarily of the sphingomyelin that cannot be degraded (Figure 17.13). Infants
with this lysosomal storage disease experience rapid and progressive
neurodegeneration as a result of deposition of sphingomyelin in the CNS, and
they die in early childhood. A less severe variant (Type B, which shows 5% or
more of normal activity) with a later age of onset and a longer survival time
causes little to no damage to neural tissue, but lungs, spleen, liver, and bone
marrow are affected, resulting in a chronic form of the disease. Although
Niemann-Pick disease occurs in all ethnic groups, Type A occurs with greater
frequency in the Ashkenazi Jewish population.

Figure 17.12 Degradation of sphingomyelin. [Note: Type B is the nonneuropathic form. It has a later age of onset and a longer survival time than Type A.]
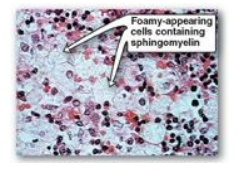
Figure 17.13 Accumulation of lipids
in spleen cells from a patient with Niemann-Pick disease.
Related Topics
