Ketone Bodies: An Alternate Fuel For Cells
| Home | | Biochemistry |Chapter: Biochemistry : Fatty Acid, Ketone Body, and Triacylglycerol Metabolism
Liver mitochondria have the capacity to convert acetyl CoA derived from fatty acid oxidation into ketone bodies.
KETONE BODIES: AN ALTERNATE FUEL FOR CELLS
Liver mitochondria have
the capacity to convert acetyl CoA derived from fatty acid oxidation into
ketone bodies. The compounds categorized as ketone bodies are acetoacetate,
3-hydroxybutyrate (also called β-hydroxybutyrate), and acetone (a
nonmetabolized side product, Figure 16.22). [Note: The two functional ketone
bodies are actually organic acids.] Acetoacetate and 3-hydroxybutyrate are
transported in the blood to the peripheral tissues. There they can be
reconverted to acetyl CoA, which can be oxidized by the TCA cycle. Ketone
bodies are important sources of energy for the peripheral tissues because 1)
they are soluble in aqueous solution and, therefore, do not need to be
incorporated into lipoproteins or carried by albumin as do the other lipids; 2)
they are produced in the liver during periods when the amount of acetyl CoA
present exceeds the oxidative capacity of the liver; and 3) they are used in
proportion to their concentration in the blood by extrahepatic tissues, such as
the skeletal and cardiac muscle, intestinal mucosa, and renal cortex. Even the
brain can use ketone bodies to help meet its energy needs if the blood levels
rise sufficiently. Thus, ketone bodies spare glucose, which is particularly
important during prolonged periods of fasting. [Note: Disorders of fatty acid
oxidation present with the general picture of hypoketosis (due to decreased
availability of acetyl CoA) and hypoglycemia (due to increased reliance on
glucose for energy.]
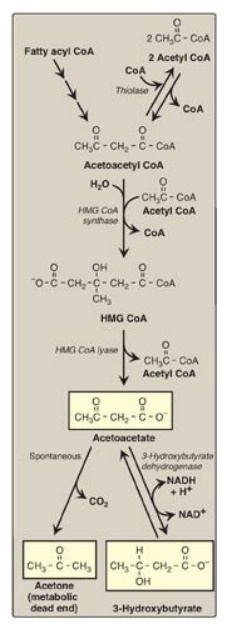
Figure 16.22 Synthesis of ketone bodies. [Note: The release of CoA in ketogenesis supports continued fatty acid oxidation.] CoA = coenzyme A; HMG = hydroxymethylglutarate; NAD(H) = nicotinamide adenine dinucleotide.
A. Synthesis of ketone bodies by the liver: ketogenesis
During a fast, the
liver is flooded with fatty acids mobilized from adipose tissue. The resulting
elevated hepatic acetyl CoA produced by fatty acid oxidation inhibits pyruvate
dehydrogenase, and activates pyruvate carboxylase. The OAA produced is used by
the liver for gluconeogenesis rather than for the TCA cycle. Therefore, acetyl
CoA is channeled into ketone body synthesis. Additionally, fatty acid oxidation
decreases the NAD+ to NADH ratio, and the rise in NADH shifts OAA to
malate. This also pushes acetyl CoA into ketogenesis (Figure 16.24).] [Note:
Acetyl CoA for ketogenesis is also generated by the catabolism of ketogenic
amino acids.]
1. Synthesis of 3-hydroxy-3-methylglutaryl coenzyme
A: The
first step, formation of acetoacetyl CoA, occurs by reversal of the thiolase
reaction of fatty acid oxidation (see Figure 16.17). Mitochondrial 3-hydroxy-3-methylglutaryl
(HMG) CoA synthase combines a third molecule of acetyl CoA with acetoacetyl CoA
to produce HMG CoA. HMG CoA synthase is the rate-limiting step in the synthesis
of ketone bodies and is present in significant quantities only in the liver.
[Note: HMG CoA is also an intermediate in cytosolic cholesterol synthesis. The
two pathways are separated by location in, and conditions of, the cell.]
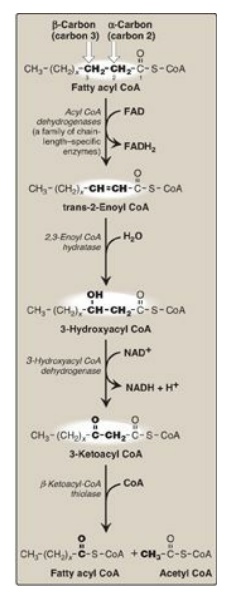
Figure 16.17 Enzymes involved in the β-oxidation of fatty acyl coenzyme A (CoA). [Note: 2,3-Enoyl CoA hydratase requires a trans double bond between carbon 2 and carbon 3.] FAD(H2) = flavin adenine dinucleotide; NAD(H) = nicotinamide adenine dinucleotide.
2. Synthesis of the ketone bodies: HMG CoA is cleaved by HMG CoA lyase to produce acetoacetate and acetyl CoA, as shown in Figure 16.22. Acetoacetate can be reduced to form 3-hydroxybutyrate with NADH as the hydrogen donor. Acetoacetate can also spontaneously decarboxylate in the blood to form acetone, a volatile, biologically nonmetabolized compound that can be released in the breath. The equilibrium between acetoacetate and 3-hydroxybutyrate is determined by the NAD+/NADH ratio. Because this ratio is low during fatty acid oxidation, 3-hydroxybutyrate synthesis is favored. [Note: The generation of free CoA during ketogenesis allows fatty acid oxidation to continue.]
B. Use of ketone bodies by the peripheral tissues: ketolysis
Although the liver
constantly synthesizes low levels of ketone bodies, their production becomes
much more significant during fasting when ketone bodies are needed to provide
energy to the peripheral tissues. 3-Hydroxybutyrate is oxidized to acetoacetate
by 3-hydroxybutyrate dehydrogenase, producing NADH (Figure 16.23). Acetoacetate
is then provided with a CoA molecule taken from succinyl CoA by succinyl
CoA:acetoacetate CoA transferase (thiophorase). This reaction is reversible,
but the product, acetoacetyl CoA, is actively removed by its conversion to two
acetyl CoAs. This pulls the reaction forward. Extrahepatic tissues, including
the brain but excluding cells lacking mitochondria (for example, RBCs),
efficiently oxidize acetoacetate and 3-hydroxybutyrate in this manner. In
contrast, although the liver actively produces ketone bodies, it lacks
thiophorase and, therefore, is unable to use ketone bodies as fuel.
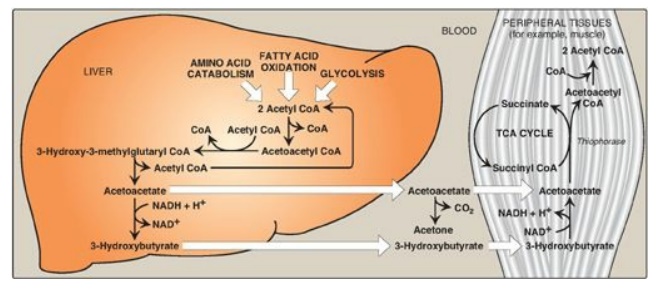
Figure 16.23 Ketone body
synthesis in the liver and use in peripheral tissues. Liver and red blood cells
cannot use ketone bodies. [Note: Thiophorase is also known as succinyl
CoA:acetoacetate CoA transferase.] CoA = coenzyme A; NAD(H) = nicotinamide
adenine dinucleotide; TCA = tricarboxylic acid.
C. Excessive production of ketone bodies in diabetes mellitus
When the rate of
formation of ketone bodies is greater than the rate of their use, their levels
begin to rise in the blood (ketonemia) and, eventually, in the urine
(ketonuria). This is seen most often in cases of uncontrolled type 1 diabetes
mellitus. In diabetic individuals with severe ketosis, urinary excretion of the
ketone bodies may be as high as 5,000 mg/24 hr, and the blood concentration may
reach 90 mg/dl (versus less than 3 mg/dl in normal individuals). A frequent
symptom of diabetic ketoacidosis (DKA) is a fruity odor on the breath, which
results from increased production of acetone. An elevation of the ketone body
concentration in the blood results in acidemia. [Note: The carboxyl group of a
ketone body has a pKa of about 4. Therefore, each ketone body loses
a proton (H+) as it circulates in the blood, which lowers the pH. Also, in DKA,
urinary loss of glucose and ketone bodies results in dehydration. Therefore,
the increased number of H+ circulating in a decreased volume of plasma can
cause severe acidosis (ketoacidosis).] Ketoacidosis may also be seen in cases
of prolonged fasting and excessive ethanol consumption.
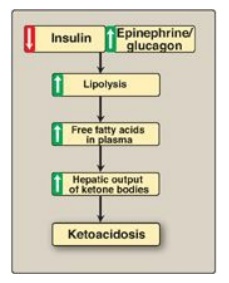
Figure 16.24 Mechanism of
diabetic ketoacidosis seen in type 1 diabetes.
Related Topics
