De Novo Synthesis of Fatty Acids
| Home | | Biochemistry |Chapter: Biochemistry : Fatty Acid, Ketone Body, and Triacylglycerol Metabolism
A large proportion of the fatty acids used by the body is supplied by the diet. Carbohydrates and protein obtained from the diet in excess of the body’s needs for these compounds can be converted to fatty acids, which are stored as TAGs.
DE NOVO SYNTHESIS OF FATTY ACIDS
A large proportion of the fatty acids used by the body is supplied by the diet. Carbohydrates and protein obtained from the diet in excess of the body’s needs for these compounds can be converted to fatty acids, which are stored as TAGs. (See : for a discussion of the metabolism of dietary nutrients in the well-fed state.) In adult humans, fatty acid synthesis occurs primarily in the liver and lactating mammary glands and, to a lesser extent, in adipose tissue. This cytosolic process incorporates carbons from acetyl coenzyme A (CoA) into the growing fatty acid chain, using adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide phosphate (NADPH).
A. Production of cytosolic acetyl coenzyme A
The first step in de
novo fatty acid synthesis is the transfer of acetate units from mitochondrial
acetyl CoA to the cytosol. Mitochondrial acetyl CoA is produced by the
oxidation of pyruvate and by the catabolism of certain amino acids. The CoA
portion of acetyl CoA, however, cannot cross the inner mitochondrial membrane,
and only the acetyl portion enters the cytosol. It does so as part of citrate
produced by the condensation of acetyl CoA with oxaloacetate (OAA) by citrate
synthase (Figure 16.6). [Note: The translocation of citrate to the cytosol
occurs when the mitochondrial citrate concentration is high. This is observed
when isocitrate dehydrogenase of the citric acid cycle is inhibited by the
presence of large amounts of ATP, causing citrate and isocitrate to accumulate.
Therefore, cytosolic citrate may be viewed as a high-energy signal. Because a
large amount of ATP is needed for fatty acid synthesis, the increase in both
ATP and citrate enhances this pathway.]
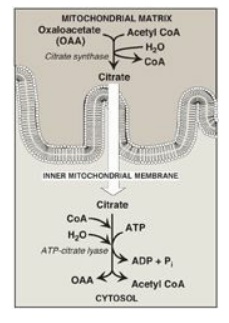
Figure 16.6 Production of cytosolic acetyl coenzyme A (CoA). Citrate is transported by the tricarboxylate transporter system. ADP = adenosine monophosphate; Pi = inorganic phosphate.
B. Carboxylation of acetyl coenzyme A to malonyl coenzyme A
The energy for the carbon-to-carbon condensations in fatty acid synthesis is supplied by the process of carboxylation followed by decarboxylation of acyl groups in the cytosol. The carboxylation of acetyl CoA to form malonyl CoA is catalyzed by acetyl CoA carboxylase (ACC) (Figure 16.7), and requires CO2 and ATP. The coenzyme is the vitamin biotin, which is covalently bound to a lysyl residue of the carboxylase (see Figure 28.16). ACC carboxylates the bound biotin, which transfers the activated carboxyl group to acetyl CoA.
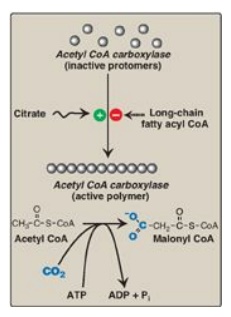
Figure 16.7 Allosteric regulation of malonyl coenzyme A (CoA) synthesis by acetyl CoA carboxylase. The carboxyl group contributed by dissolved CO2 is shown in blue. Pi = inorganic phosphate; ADP = adenosine diphosphate.
1. Short-term regulation of acetyl coenzyme A
carboxylase: This
carboxylation is both the rate-limiting and the regulated step in fatty acid
synthesis (see Figure 16.7). The inactive form of ACC is a protomer. The enzyme
undergoes allosteric activation by citrate, which causes protomers to
polymerize, and allosteric inactivation by long-chain fatty acyl CoA (the end
product of the pathway), which causes depolymerization. A second mechanism of
short-term regulation is by reversible phosphorylation. Adenosine
monophosphate–activated protein kinase (AMPK) phosphorylates and inactivates
ACC. AMPK itself is allosterically activated by AMP and covalently activated by
phosphorylation via several kinases. At least one of these AMPK kinases is
activated by cAMP-dependent protein kinase A (PKA). Thus, in the presence of
counterregulatory hormones, such as epinephrine and glucagon, ACC is
phosphorylated and, thereby, inactivated ( Figure 16.8). In the presence of
insulin, ACC is dephosphorylated and, thereby, activated. [Note: This is
analogous to the regulation of glycogen synthase.]
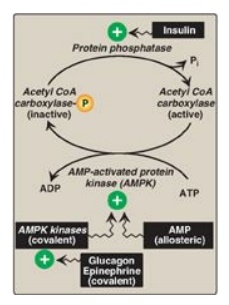
Figure 16.8 Covalent regulation (phosphorylation) of acetyl CoA carboxylase by AMPK, which itself is regulated both covalently and allosterically. CoA = coenzyme A; ADP = adenosine diphosphate; P = phosphate; Pi = inorganic phosphate; AMP = adenosine monophosphate.
2. Long-term regulation of acetyl coenzyme A carboxylase: Prolonged consumption of a diet containing excess calories (particularly high-calorie, high-carbohydrate diets) causes an increase in ACC synthesis, thereby increasing fatty acid synthesis. Conversely, a low-calorie or a high-fat diet causes a reduction in fatty acid synthesis by decreasing ACC synthesis. [Note: Synthesis of the carboxylase is upregulated by insulin via a sterol regulatory element–binding protein, SREBP-1. The function and regulation of SREBPs. Fatty acid synthase (see below) is similarly regulated by diet and SREBP-1.] Metformin, used in the treatment of type 2 diabetes, lowers serum TAG through activation of AMPK, resulting in inhibition of ACC activity (by phosphorylation) and inhibition of ACC and fatty acid synthase expression (by decreasing SREBP-1). Metformin also lowers blood glucose by increasing AMPK-mediated uptake of glucose by muscle.
C. Fatty acid synthase: a multifunctional enzyme in eukaryotes
The remaining series of
reactions of fatty acid synthesis in eukaryotes is catalyzed by the
multifunctional, dimeric enzyme, fatty acid synthase (FAS). Each FAS monomer is
a multicatalytic polypeptide with seven different enzymic domains plus a domain
that covalently binds a molecule of 4I
-phosphopantetheine. [Note: 4I
-Phosphopantetheine, a derivative of the vitamin pantothenic acid, carries acyl
units on its terminal thiol (–SH) group during fatty acid synthesis. It also is
a component of CoA.] In prokaryotes, FAS is a multienzyme complex, and the 4I-phosphopantetheine domain is a
separate protein, referred to as the acyl carrier protein (ACP). ACP is used to
refer to the phosphopantetheine-containing domain of eukaryotic FAS. The
reaction numbers in brackets below refer to Figure 16.9.
[1]. An acetyl group is
transferred from acetyl CoA to the –SH group of the ACP. Domain: Acetyl CoA-ACP
acetyltransacylase.
[2]. Next, this
two-carbon fragment is transferred to a temporary holding site, the thiol group
of a cysteine residue on the enzyme.
[3]. The now-vacant ACP
accepts a three-carbon malonyl group from malonyl CoA. Domain: Malonyl CoA-ACP
transacylase.
[4]. The acetyl group on the cysteine residue condenses with the malonyl group on ACP as the CO2 originally added by acetyl CoA carboxylase is released. The result is a four-carbon unit attached to the ACP domain. The loss of free energy from the decarboxylation drives the reaction. Domain: 3-Ketoacyl-ACP synthase, also known as “condensing enzyme.”
The next three
reactions convert the 3-ketoacyl group to the corresponding saturated acyl
group by a pair of NADPH-requiring reductions and a dehydration step.
[5] The keto group is
reduced to an alcohol. Domain: 3-Ketoacyl-ACP reductase.
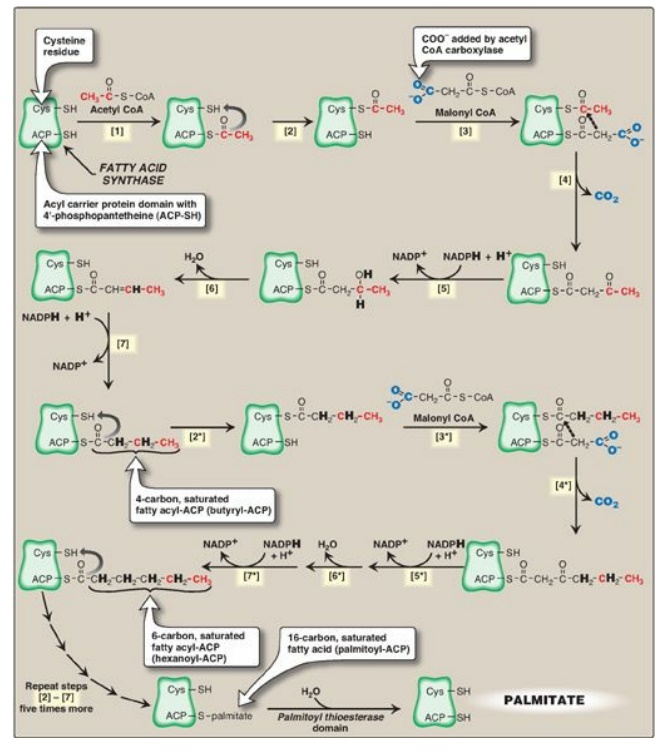
Figure 16.9 Synthesis of
palmitate (16:0) by multifunctional fatty acid synthase (FAS). [Note: Numbers
in brackets correspond to bracketed numbers in the text. A second repetition of
the steps is indicated by numbers with an asterisk (*).
Carbons provided directly by acetyl coenzyme A
(CoA) are shown in red.] Cys = cysteine; ACP = acyl carrier protein domain;
NADP(H) = nicotinamide adenine dinucleotide phosphate.
[6]. A molecule of
water is removed, creating a double bond between carbons 2 and 3 (the α- and
β-carbons). Domain: 3-Hydroxyacyl-ACP dehydratase.
[7] The double bond is
reduced. Domain: Enoyl-ACP reductase.
The result of these seven steps is production of a four-carbon compound (butyryl) whose three terminal carbons are fully saturated, and which remains attached to the ACP domain. These seven steps are repeated, beginning with the transfer of the butyryl chain from the ACP to the cysteine residue [2*], the attachment of a molecule of malonate to the ACP [3*], and the condensation of the two molecules liberating CO2 [4*]. The carbonyl group at the β-carbon (carbon 3, the third carbon from the sulfur) is then reduced [5*], dehydrated [6*], and reduced [7*], generating hexanoyl-ACP. This cycle of reactions is repeated five more times, each time incorporating a two-carbon unit (derived from malonyl CoA) into the growing fatty acid chain at the carboxyl end. When the fatty acid reaches a length of 16 carbons, the synthetic process is terminated with palmitoyl-S-ACP. [Note: Shorter-length fatty acids are important end products in the lactating mammary gland.] Palmitoyl thioesterase, the final catalytic activity of FAS, cleaves the thioester bond, releasing a fully saturated molecule of palmitate (16:0). [Note: All the carbons in palmitic acid have passed through malonyl CoA except the two donated by the original acetyl CoA, which are found at the methyl (ω) end of the fatty acid. This underscores the rate-limiting nature of the ACC reaction.]
D. Major sources of the reductant required for fatty acid synthesis
The pentose phosphate
pathway is a major supplier of NADPH, the reducatant required for fatty acid
synthesis. Two NADPH are produced for each molecule of glucose that enters this
pathway. The cytosolic conversion of malate to pyruvate, in which malate is oxidized
and decarboxylated by cytosolic malic enzyme (NADP+-dependent malate
dehydrogenase), also produces cytosolic NADPH (and CO2) as shown in
Figure 16.10. [Note: Malate can arise from the reduction of OAA by cytosolic
NADH-dependent malate dehydrogenase (see Figure 16.10). One source of the
cytosolic NADH required for this reaction is that produced during glycolysis.
OAA, in turn, can arise from citrate. Recall from Figure 16.6 that citrate,
formed from OAA and acetyl CoA by citrate synthase, was shown to move from the
mitochondria into the cytosol, where it is cleaved into acetyl CoA and OAA by
ATP-citrate lyase.] A summary of the interrelationship between glucose
metabolism and palmitate synthesis is shown in Figure 16.11.
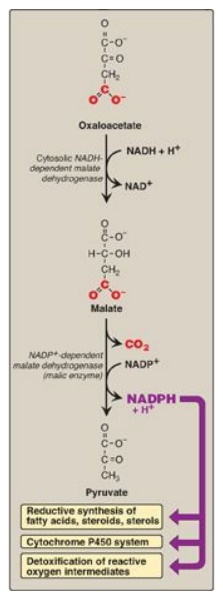
Figure 16.10 Cytosolic conversion of oxaloacetate to pyruvate with the generation of nicotinamide adenine dinucleotide phosphate (NADPH). [Note: The pentose phosphate pathway is also a source of NADPH.] NAD(H) = nicotinamide adenine dinucleotide.
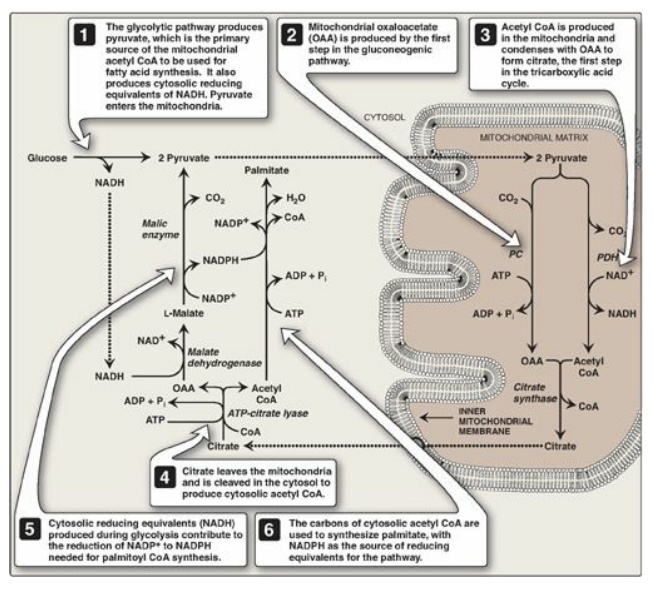
Figure 16.11 Interrelationship between glucose metabolism and palmitate synthesis. CoA = coenzyme A; NAD(H) = nicotinamide adenine nucleotide; NADP(H) =nicotinamide adenine dinucleotide phosphate; ADP = adenosine diphosphate; Pi = inorganic phosphate; PC = pyruvate carboxylase; PDH = pyruvate dehydrogenase.
E. Further elongation of fatty acid chains
Although palmitate, a 16-carbon, fully saturated LCFA (16:0), is the primary end product of fatty acid synthase activity, it can be further elongated by the addition of two-carbon units to the carboxylate end in the smooth endoplasmic reticulum (SER). Elongation requires a system of separate enzymes rather than a multifunctional enzyme. Malonyl CoA is the two-carbon donor, and NADPH supplies the electrons. The brain has additional elongation capabilities, allowing it to produce the very-long-chain fatty acids ([VLCFAs] over 22 carbons) that are required for synthesis of brain lipids.
F. Desaturation of fatty acid chains
Enzymes (desaturases)
also present in the SER are responsible for desaturating LCFAs (that is, adding
cis double bonds). The desaturation reactions require O2, NADH,
cytochrome b5, and its FAD-linked reductase. The fatty acid and the NADH get
oxidized as the O2 gets reduced to H2O. The first double
bond is typically inserted between carbons 9 and 10, producing primarily oleic
acid, 18:1(9), and small amounts of palmitoleic acid, 16:1(9). A variety of
polyunsaturated fatty acids can be made through additional desaturation
combined with elongation.
Humans have carbon 9, 6, 5, and 4 desaturases but
lack the ability to introduce double bonds from carbon 10 to the ω end of the
chain. This is the basis for the nutritional essentiality of the
polyunsaturated acids ω-6 linoleic and ω-3 linolenic.
G. Storage of fatty acids as components of triacylglycerols
Mono-, di-, and
triacylglycerols consist of one, two, or three molecules of fatty acid
esterified to a molecule of glycerol. Fatty acids are esterified through their
carboxyl groups, resulting in a loss of negative charge and formation of
“neutral fat.” [Note: If a species of acylglycerol is solid at room temperature,
it is called a fat, whereas if it is liquid, it is called an oil.]
1. Structure: The three fatty acids esterified to a glycerol
molecule to form a TAG are usually not of the same type. The fatty acid on
carbon 1 is typically saturated, that on carbon 2 is typically unsaturated, and
that on carbon 3 can be either. Recall that the presence of the unsaturated
fatty acid(s) decrease(s) the Tm of the lipid. An example of a TAG molecule is
shown in Figure 16.12.
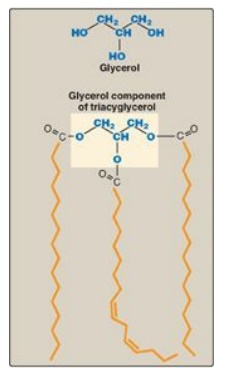
Figure 16.12 A triacylglycerol
with an unsaturated fatty acid on carbon 2. Orange denotes the hydrophobic
portions of the molecule.
2. Storage: Because TAGs are only slightly soluble in water
and cannot form stable micelles by themselves, they coalesce within white
adipocytes to form large oily droplets that are nearly anhydrous. These
cytosolic lipid droplets are the major energy reserve of the body. [Note: TAGs
stored in brown adipocytes serve as a source of heat through nonshivering
thermogenesis.]
3. Synthesis of glycerol 3-phosphate: Glycerol 3-phosphate is the
initial acceptor of fatty acids during TAG synthesis. There are two pathways
for its production (Figure 16.13). In both liver (the primary site of TAG
synthesis) and adipose tissue, glycerol 3-phosphate can be produced from
glucose, using first the reactions of the glycolytic pathway to produce
dihydroxyacetone phosphate ([DHAP]). DHAP is reduced by glycerol 3-phosphate
dehydrogenase to glycerol 3-phosphate. A second pathway found in the liver, but
not in adipose tissue, uses glycerol kinase to convert free glycerol to
glycerol phosphate (see Figure 16.13). [Note: The glucose transporter in
adipocytes (GLUT-4) is insulin dependent. Thus, when plasma glucose (and,
therefore, plasma insulin) levels are low, adipocytes have only a limited
ability to synthesize glycerol phosphate and cannot produce TAG de novo.]
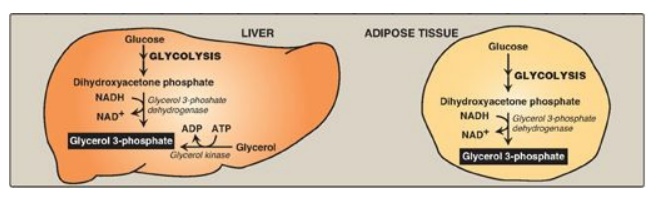
Figure 16.13 Pathways for
production of glycerol 3-phosphate in liver and adipose tissue. [Note: Glycerol
3-phosphate can also be generated by glyceroneogenesis.] NAD(H) = nicotinamide
adenine dinucleotide; ADP = adenosine diphosphate.
4. Activation of a free fatty acid: A fatty acid must be converted to
its activated form (bound to CoA) before it can participate in metabolic
processes such as TAG synthesis. This reaction, illustrated in Figure 15.6, is
catalyzed by a family of fatty acyl CoA synthetases (thiokinases).
5. Synthesis of triacylglycerol from glycerol
3-phosphate and fatty acyl coenzyme As: This pathway involves four reactions, shown in
Figure 16.14. These include the sequential addition of two fatty acids from
fatty acyl CoAs, the removal of phosphate, and the addition of the third fatty
acid.
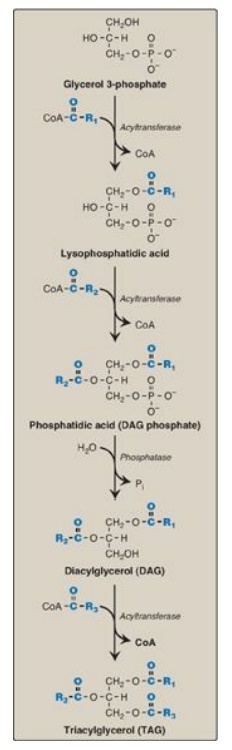
Figure 16.14 Synthesis of TAG. R1-R3 = activated fatty acids. CoA = coenzyme A; Pi = inorganic phosphate.
H. Different fates of triacylglycerol in liver and adipose tissue
In WAT, TAG is stored in a nearly anhydrous form as fat droplets in the cytosol of the cells. It serves as “depot fat,” ready for mobilization when the body requires it for fuel. Little TAG is stored in healthy liver. Instead, most is exported, packaged with other lipids and apolipoproteins to form lipoprotein particles called very-low-density lipoproteins (VLDLs). Nascent VLDLs are secreted directly into the blood where they mature and function to deliver the endogenously derived lipids to the peripheral tissues. [Note: Recall from Chapter 15 that chylomicrons carry dietary (exogenously derived) lipids.] Plasma lipoproteins are discussed in Chapter 18.
Related Topics
