Overview of Phospholipids
| Home | | Biochemistry |Chapter: Biochemistry : Phospholipid, Glycosphingolipid, and Eicosanoid Metabolism
Phospholipids are polar, ionic compounds composed of an alcohol that is attached by a phosphodiester bond to either diacylglycerol (DAG) or sphingosine.
OVERVIEW OF PHOSPHOLIPIDS
Phospholipids are polar,
ionic compounds composed of an alcohol that is attached by a phosphodiester
bond to either diacylglycerol (DAG) or sphingosine. Like fatty acids,
phospholipids are amphipathic in nature. That is, each has a hydrophilic head,
which is the phosphate group plus whatever alcohol is attached to it (for
example, serine, ethanolamine, and choline, highlighted in blue in Figure 17.1A
), and a long, hydrophobic tail containing fatty acids or fatty acid–derived
hydrocarbons (shown in orange in Figure 17.1A). Phospholipids are the
predominant lipids of cell membranes. In membranes, the hydrophobic portion of
a phospholipid molecule is associated with the nonpolar portions of other
membrane constituents, such as glycolipids, proteins, and cholesterol. The
hydrophilic (polar) head of the phospholipid extends outward, interacting with
the intracellular or extracellular aqueous environment (see Figure 17.1A ).
Membrane phospholipids also function as a reservoir for intracellular
messengers, and, for some proteins, phospholipids serve as anchors to cell
membranes. Nonmembrane phospholipids serve additional functions in the body,
for example, as components of lung surfactant and essential components of bile,
where their detergent properties aid in the solubilization of cholesterol.
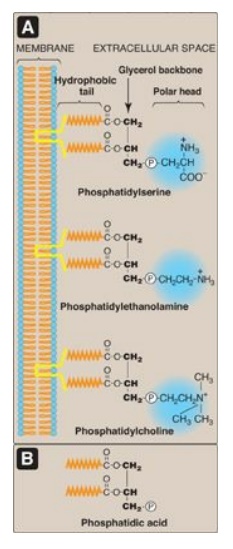
Figure 17.1 A. Structures of
some glycerophospholipids. B. Phosphatidic acid. P
= phosphate (an anion).
Related Topics
