The Operation of Mills
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Size, Reduction and Classification
In some operations, such as those in which ores are processed, size reduction may constitute a major proportion of total process costs.
THE OPERATION OF MILLS
In
some operations, such as those in which ores are processed, size reduction may
constitute a major proportion of total process costs. The efficiency with which
energy is utilized is, therefore, of great importance. Drugs, on the other
hand, fall into a class of materials that are high in cost and are processed in
relatively small quantities. The contribution of grinding to total costs is,
there-fore, smaller, and the choice of machine can usually be made on
technological rather than economic grounds. Generally, drugs are easy to grind.
The operation is classified as fine grinding if the bulk of the product passes
a 200-mesh screen (7.6 x 10-5 m) or as superfine grinding if a
powder of a few microns or less is required. Most pharmaceutical grinding falls
into these classes, although coarser grinding is applied to vegetable drugs
before extraction.
Heywood
has stated that any type of crushing or grinding machine exhibits optimal
comminution conditions for which the ratio of the energy to new surface is
minimal (Heywood, 1957). If finer grinding is attempted in such a machine, the
ratio will be increased. Mills may thus become grossly inefficient if called
upon to grind at a size for which they were not designed. A limited size
reduction ratio is imposed upon a single operation, larger ratios being
obtained by the adoption of several stages, each employing a suitable mill. The
fluid energy mill, which presents a size reduction ratio of up to 400, is
exceptional.
A
low retention time is inherent in free-crushing machines. Little over-grinding
takes place, and the production of excessive undersize material or “fines” is
avoided. Protracted milling times are found with many slow-speed mills, with
the result that considerable overgrinding takes place. Accumulation of product
particles within the mill reduces the effectiveness of breaking stresses, and
the efficiency of milling progressively decreases. This is typical of
“open-circuit” grinding, in which the material is passed only once through the
mill, remaining until virtually all has reached the required product size. An
overall increase in efficiency is secured in “closed-circuit” grinding. Product
particles are removed from the mill by means of a current of air or liquid or,
alternatively, by screens. The removed product may then be classified and any
oversize material returned to the mill. Adoption of closed-circuit grinding
techniques is only possible on a relatively large scale. On a smaller scale,
the effect can be simulated by periodic classification of the entire mill
contents and the removal of material that has reached the required size.
Dry and Wet Grinding
Between
the approximate limits of 5% and 50% moisture, materials cake and do not flow.
Both factors oppose effective grinding. Dry grinding is carried out at low
moisture contents, the upper limit depending on the nature of the material.
Although 5% or more moisture may be permissible for vegetable drugs, it would
prove excessive during the milling of a coarse, impervious solid.
Wet
grinding is a common procedure when a fluid suspension is required and drying,
which would prove a significant drawback, is unnecessary. An excellent
dispersion can be produced simultaneously, and in some operations, this
provides the primary objective, size reduction being of secondary impor-tance.
Wet grinding may also be adopted when the size reduction achieved during dry
grinding is prematurely limited by aggregation.
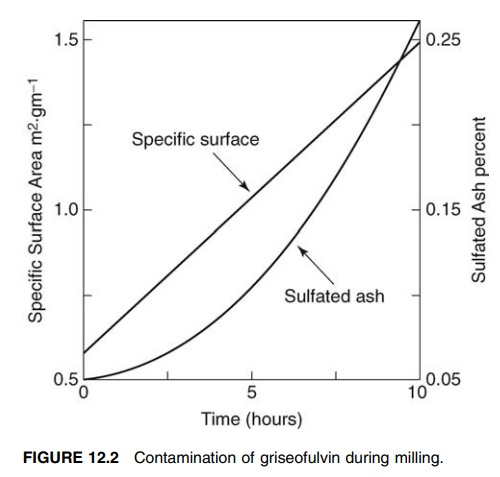
FIGURE 12.2 Contamination of griseofulvin during milling.
Certain
general advantages are secured during wet grinding. These include an increased
mill capacity, a lower energy consumption, the elimination of hazards from
dust, and easier handling of materials. The principal disad-vantage, apart from
the possible inclusion of a drying stage, is the increased wear of the grinding
medium.
Contamination
Wear
of grinding elements, which occurs in all mills, results in the contamina-tion
of the product. This factor influences the choice of constructional materials,
and ceramics and stainless steel are most commonly used. Contamination is
normally slight. However, in the protracted periods often associated with the
production of very fine powders, it may become severe. This is illustrated by
the data presented in Figure 12.2, which shows a progressive increase in a
sulfated ash value of the material due to wear of the ceramic mill.
Closed
mills, preventing the ingress of bacteria, must be used for grinding sterile
materials.
Temperature Sensitivity
Care
must be exercised during the milling of temperature-sensitive materials,
especially when a very fine product is required. Caking results if the
softening point is exceeded. Materials may be chilled before grinding, or
facilities for cooling the mill during grinding may be provided. Waxy solids
can be suc-cessfully ground with dry ice, the low temperatures conferring
brittle charac-teristics on the material. Chemical degradation may occur at
high grinding temperatures. Oxidative changes can be prevented by grinding in
an inert atmosphere such as nitrogen.
Structural Changes
Several
examples of change of physical structure during very fine grinding have been
reported. Gammage and Glasson found changes in the crystal form of calcium
carbonate after ball milling (Gammage and Glasson, 1963). Distortion of the kaolinite
lattice during very fine grinding was reported by Gregg (Gregg, 1955).
Cleverley and Williams found that various barbiturate polymorphs were formed
during grinding (Cleverley and Williams, 1959). Changes such as these could
affect solubility and other physical characteristics, which, in turn, might
influence formulation and therapeutic value.
Dust Hazards
Hazards
from dust may become acute during dry grinding. Extremely potent materials
require dust-proofing of machines and the supply of dust-proof clothing and
masks to operators. Danger may also arise from the explosive nature of many
dusts.
Related Topics
