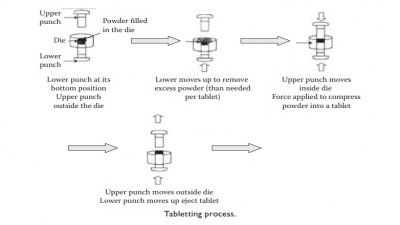
Types of tablets
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Tablets
Depending on the physicochemical properties of the drug, site and extent of drug absorption in the gastrointestinal (GI) tract, stability to heat, light, or moisture, biocompatibility with other ingredients, solubility, and dose.
Types of tablets
Depending
on the physicochemical properties of the drug, site and extent of drug
absorption in the gastrointestinal (GI) tract, stability to heat, light, or
moisture, biocompatibility with other ingredients, solubility, and dose, the
following types of tablets are commonly formulated (Table
20.1):
1. Swallowable tablets
The
most common types of tablets are swallowed whole. These tablets dis-integrate
and release their contents in the GI tract.
2. Effervescent tablets
These
tablets are formulated to allow dissolution or dispersion in water prior to
administration and should not be swallowed whole. In addition to the DS, these
tablets contain sodium carbonate or bicarbonate and an organic acid such as
tartaric acid. In the presence of water, these additives react, liberat-ing
carbon dioxide, which acts as a disintegrator and produces effervescence. The
drug is released into the aqueous medium as a solution, if it is highly
soluble, or suspension. Ingestion of a dissolved or finely dispersed drug
pro-vides a rapid rate of drug absorption. Therefore, effervescent tablets can
be suitable for acute conditions that require immediate relief, such as pain
and gastric acidity. For example, cephalon’s fentanyl effervescent tablet can
be used to reduce the intensity of breakthrough pain in cancer patients.
3. Chewable tablets
Chewable
tablets are used when a faster rate of dissolution and/or buc-cal absorption is
desired. Chewable tablets consist of the drug dispersed throughout a saccharide
base that provides mild sweetness. Flavors, sweet-eners, and colors are also
added to chewable tablets to improve palatability and organoleptic appeal. The
drug is released from the dosage form by physical disruption associated with
chewing and dissolution in the fluids of the oral cavity, and the presence of a
effervescent material. For example, some antacid tablets can be chewed to
obtain quick indigestion relief. Chewable tablets are typically prepared by
compression and usually contain mannitol or sorbitol as saccharide, mildly
sweet, fillers. Mannitol is some-times preferred as a chewable base diluent,
because it has a pleasant cooling
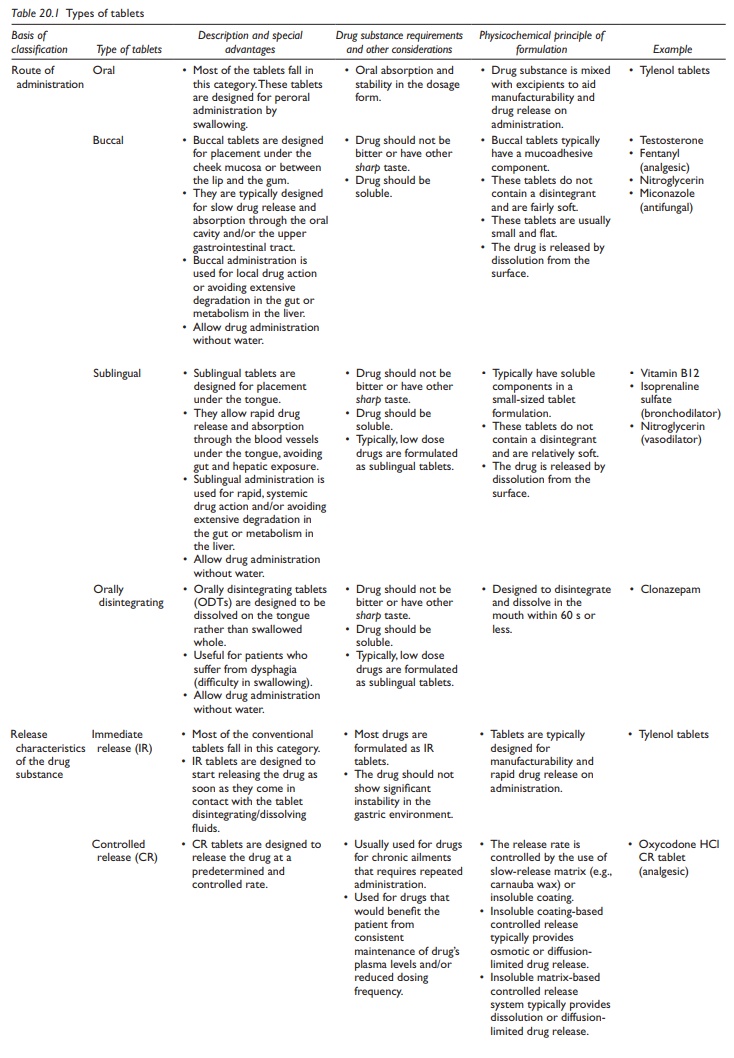
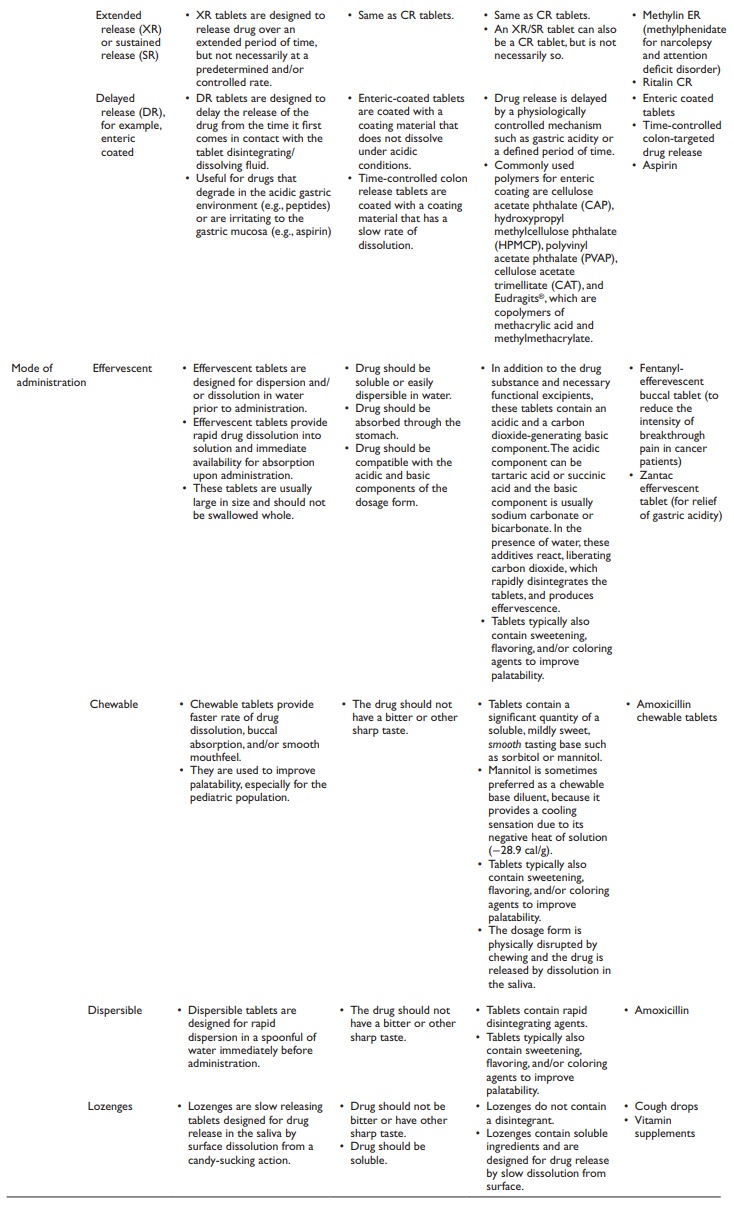
Table 20.1 Types of tablets
4. Buccal and sublingual tablets
Buccal
and sublingual tablets dissolve in the cheek pouch (buccal) or under the tongue
(sublingual). Buccal or sublingual route of drug absorption bypasses hepatic
metabolism, often referred to as the first-pass effect on oral administra-tion,
and is preferred for low dose drugs that have extensive hepatic metabo-lism.
Sublingual administration also allows rapid drug absorption, which may be
critical in cases such as nitroglycerin for chronic heart failure. Other
exam-ples include isoprenaline sulfate (bronchodilator), glyceryl trinitrate
(vasodila-tor), and testosterone tablets. These tablets are usually small and
flat, do not contain a disintegrant, and are intended for dissolution in the
local fluids.
5. Lozenges
Lozenges
are slow dissolving compressed tablets that do not contain a disin-tegrant.
Some lozenges contain antiseptics (e.g., benzalkonium) or antibiotics for local
effects in the mouth. Lozenges are also used for systemic effect, such as those
containing vitamin supplements. Lozenges are palatable and organo-leptically
appealing by the addition of flavors, sweeteners, and colors.
6. Coated tablets
Most
tablets are coated for one or more of the following reasons:
·
To prevent decomposition of drugs sensitive to air (oxygen),
light, or humidity
·
To minimize the unpleasant taste of certain drugs that may
come dur-ing partial dissolution of the drug in buccal fluids during absorption
·
To improve swallowability and palatability by increasing
surface smoothness in the mouth
·
To provide visual appeal and consistency, smooth surface
texture, and uniform distribution of color
·
To serve as anticounterfeiting medium by incorporating
tracer com-pounds in the coating material
·
To allow containment of highly potent compounds in the core
of the tablet and, thus, avoid exposure to personnel handling of the tablets
Coating
is not used on buccal, sublingual, chewable, effervescent, or dis-persible
tablets to avoid any delay in drug release due to the time required for the
rupture or dissolution of the coating material (Table
20.2).
Coating
of core (compressed, uncoated) tablets is carried out by load-ing the tablets
in a moving, perforated pan supplied with dry hot air and spraying the coating
dispersion onto the tablet bed at a rate matched with the rate of evaporation
of the solvent. This leads to the deposition of a film
Table 20.2 Characteristics of types of tablet coatings
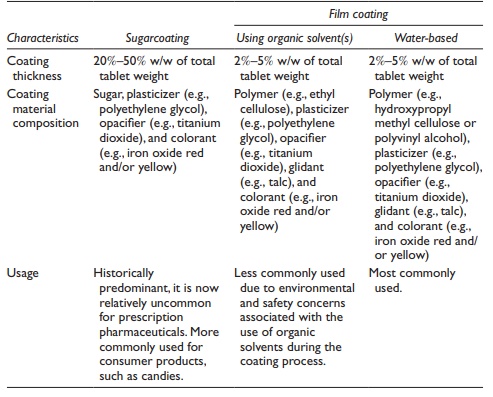
This process is
carried out for a sufficient duration of time to allow uniform and elegant
coverage of the entire surface of the tablet by the coating material. The
coating material consists of an opacifier (such as fine particle size titanium
dioxide), color, plasticizer (such as polyethylene glycol), and a polymer (such
as hydroxypropyl methylcellulose [HPMC] or polyvinyl alcohol [PVA]). Typically,
about 3% w/w application of the coating material provides com-plete coverage by
the formation of a thin film around the tablet. The coated tablets are called film-coated tablets.
Other
types of coated tablets are sugarcoated, gelatin-coated (gel caps), and
enteric-coated tablets. Sugarcoated tablets are produced by the application of
sucrose solution, containing preservatives, colorants, sweeteners, and flavors,
to the core with a relatively high (~30% w/w) weight buildup. Film coating has
almost completely taken over sugar coating in the pharmaceutical industry
because of shorter processing time and the smaller size of the coated tablet. A
common example of sugarcoated tablet is the M&Ms, which are consist of a
solidified liquid-chocolate center and a hard-candy shell which is a
combination of sugar and corn syrup. Characteristics of sugarcoated and
film-coated tablets are summarized in Table 20.3.
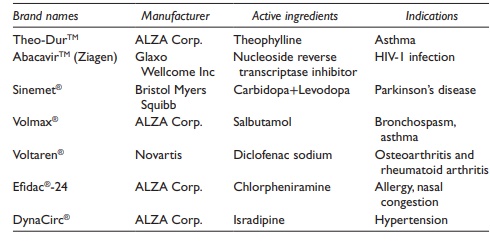
Table 20.3 Examples of sustained release tablets
Gelatin-coated
tablets, commonly known as gelcaps, are capsule-shaped compressed tablets
coated with a gelatin layer. These tablets are produced by dipping the core
tablets in a solution of gelatin with colors and preser-vatives, followed by
drying. This allows the product to be smaller than an equivalent capsule filled
with an equivalent amount of powder, and pro-vides an elegant visual appeal.
Many over-the-counter (OTC) medications are marketed as gelcaps.
7. Enteric-coated tablets
GI
fluid pH increases progressively from acidic to basic from the stomach through
the intestines to the colon. The changes in GI pH can be utilized to release a
drug at a particular physiological location in the GI tract. In particular,
oral solid dosage forms can be coated with a polymer that is insoluble at the
acidic stomach pH and soluble at basic intestinal pH. Such polymers are known
as enteric polymers and such coatings are termed enteric coatings.
Enteric-coated tablets are the tablets coated with enteric polymers. Complete
coating of the tablet with these polymers allows the polymer to form a barrier
between the core of the tablet and the surround-ing aqueous medium. Thus, the
enteric-coated tablet remains insoluble in the low pH environment of the
stomach, but dissolves readily on passage into the small intestine with its
elevated pH.
Enteric
coating is used to minimize irritation of the gastric mucosa by certain drugs
and/or protect sensitive drugs against decomposition in the acidic environment
of the stomach. For example, aspirin produces less gas-tric bleeding when
formulated as enteric-coated sustained-release (SR) tab-lets than conventional
immediate release dosage forms.
Commonly
used polymers for enteric coating are acid-impermeable poly-mers, such as
cellulose acetate phthalate (CAP), HPMC phthalate (HPMCP), polyvinyl acetate
phthalate (PVAP), and Eudragits®, which are copolymers of methacrylic acid and
methylmethacrylate. Eudragits are available in different grades. The drug
release behavior of a Eudragit-coated tablet is controlled through the ratio of
methacrylic acid copolymers. For example, the ratio of carboxyl to ester groups
is approximately 1:1 in fast dissolving Eudragit L100 and 1:2 in slow
dissolving Eudragit S100.
8. Immediate release tablets
Most
tablets (discussed earlier) are immediate release (IR) tablets, that is, they
make all the drugs available to the dissolution medium immediately on coming in
contact with the aqueous medium. The drug dissolves at a rate determined by the
composition of the dissolution medium (such as pH) and physicochemical
properties of the drug (such as solubility and particle size).
9. Controlled release tablets
In
contrast to the IR tablets, certain dosage forms, such as controlled-release
(CR) or extended-release (XR) tablets, are designed to control or extend,
respectively, the rate at which drug dissolves in the aqueous medium. Thus, CR
tablets reduce the rate of drug release to a slow, controlled rate, which is
typically zero order. XR tablets, on the other hand, extend the duration of
drug release by slowing down the rate of drug release but may not have control
on the rate (i.e., may not provide zero-order kinetics of drug release). CR or
XR tablets are sometimes also called SR tablets.
CR
tablets can reduce dosing frequency, increase patient compliance, and may
reduce side effects of certain drugs. The rate-controlling feature of the CR
tablets could be either the matrix or the film coating. Coformulating or mixing
drugs with water-insoluble polymers prepare matrix tablets. A slow dissolving
polymer matrix, such as high molecular weight HPMC, can be used to prepare SR tablets
of highly water-soluble drugs. On coming in contact with the aqueous
dissolution medium, the core tablet dissolves by surface erosion, and the rate
of surface erosion is controlled by the rate of dissolution of the polymer
matrix. For example, metformin hydrochloride XR tablets (Glucophage XR
tablets®) are matrix tablets.
Membrane-coated
SR tablets utilize an insoluble membrane to reduce or control the rate of drug
release. The insoluble membrane can be impreg-nated with a soluble polymer to
provide pores from which the drug can diffuse out or have a hole for
osmotically CR tablets. Often, a combination of IR and SR components is
included in a dosage form to provide a loading dose (by the IR component)
followed by a slow releasing maintenance dose (by the SR component). The IR and
the SR component can form two dif-ferent layers of a bilayer tablet, for
example. These components can also be mixed together and packaged in a capsule.
The SR and IR components can also be compressed together. For example, Theo-Dur®
CR tablet of theoph-ylline consists of two components: (1) a matrix of
compressed theophylline crystals and (2) coated theophylline granules embedded
in the matrix. In contact with fluids in GI tract, theophylline diffuses slowly
through the wall of the free granules, which dissolves with time. After oral
administra-tion of Theo-Dur® 300 mg tablets to human subjects, serum
theophylline concentrations over 1 mg/ml were maintained over 24 hours.
Examples
of commonly used SR drug delivery products are listed in Table 20.3.
Related Topics