Hard gelatin capsules
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Capsules
Gelatin is a colorless, almost tasteless, translucent proteinaceous substance that is brittle when dry and elastic when mixed with controlled amount of moisture.
Hard gelatin
capsules
Gelatin
is a colorless, almost tasteless, translucent proteinaceous substance that is
brittle when dry and elastic when mixed with controlled amount of moisture. It
is produced by irreversible, partial hydrolysis of collagen, which is obtained
from animal skin and bones. It forms a semisolid col-loid gel in the presence
of water, which displays a temperature-dependent gel–sol transformation and
viscoelastic flow. It has crystallites (microscopic crystals formed during the
cooling phase of manufacture of capsule shells) that stabilize the
three-dimensional gel network structure and are respon-sible for streaming
birefringence in gelatin solutions.
A
hard gelatin capsule shell consists of two pieces: a cap and a body. The body
has slightly lower diameter than the cap and fits inside the cap. They are
produced empty and are then filled in a separate operation. During the capsule
filling unit operation, the body is filled with the medicament, fol-lowed by
the insertion of the cap over the body.
The
shapes and interlocking arrangement of the body and the cap have evolved to
meet the manufacturing and use requirements of hard gelatin capsules as shown
in Figure 21.2.
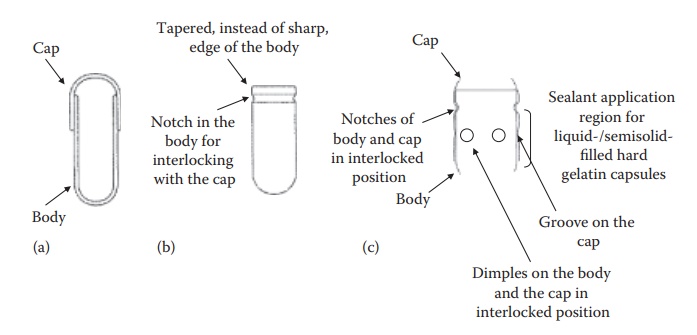
Figure 21.2 Schematic diagrams
(a–c) of hard gelatin capsules illustrating their design features. The larger,
narrower part of the capsules is the body and the smaller, wider part is the
cap.
·
Conventionally, the body and the cap had smooth edges with a
diam-eter of the cap being slightly higher than that of the body. The two
components could slide over each other (Figure 21.2a).
·
To minimize defects during the production process, the
design of the edge of the body was tapered to allow smooth penetration into the
cap with minimum defects during high-speed production operation (Figure 21.2b).
·
The capsules were modified to have an encircling groove each
on the cap and the body (Figure 21.2c) and/or a
notch to allow firm locking of the cap on the body (Figure
21.2b and c).
·
To accommodate the need for a firm seal in the case of
liquid and semisolid-filled hard gelatin capsules, raised circular bands (dimples) were introduced on the body and
the cap along the sealing zone (Figure 21.2c).
·
For the use of hard gelatin capsules in double-blind
clinical trials, it was necessary to have hard gelatin capsules that could not
be reopened after closing. To meet this objective, capsules with the cap that
covers most of the body were developed.
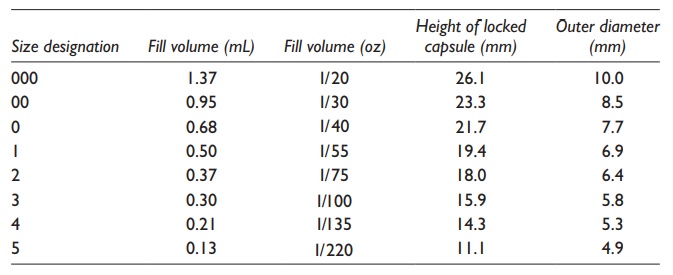
For
human use, empty gelatin capsules are manufactured in eight sizes, ranging from
000 (the largest, fill volume 1.37ml) to 5 (the smallest, fill volume 0.13ml),
as shown in Table 21.1. The powder-filling
capacity of these capsules varies depending on the packed density of the
formulation. Modern high-speed capsule-filling machines are capable of filling
up to 200,000 capsules per hour, matching the production capacity of tablets.
The formulation filled weight in the capsules can range from 30 to 1400 mg,
depending on the powder’s bulk and compact densities.
Table 21.1 Typical sizes of hard gelatin capsules
Hard
gelatin capsules can be filled with powders, granules, pellets, microtablets,
tablets, capsules, liquids, or semisolids. Most of the marketed products
contain powders or granules. Recently, the liquid- or semisolid-filled hard
gelatin capsules have gained popularity.
After
ingestion, the gelatin shell imbibes water, softens, swells, and dissolves in
the GI tract. Encapsulated drugs are released rapidly and dis-persed easily,
leading to rapid absorption.
Advantages and disadvantages of hard gelatin capsules
Comparison with tablets
Hard
gelatin capsules often provide formulation capability for uniquely challenging
drug molecules. For example, a drug candidate with a low melt-ing point or that
is liquid at room temperature usually has poor manufac-turability as a tablet,
especially if it requires a high dose. Such a compound can be encapsulated in a
liquid- or semisolid-filled hard gelatin capsule.
In
addition, very low-dose drugs (in μg) can have content uniformity
chal-lenges when formulated as a tablet. The distribution of these drugs can be
significantly better when encapsulated as a solution in a liquid or semisolid
matrix in a hard gelatin capsule.
Hard
gelatin capsules generally require less formulation components and place less
stringent requirement on the powder properties of the formula-tion. They can
also allow flexibility in formulation with the possibility of filling one or
more of diverse systems including powders, granules, pellets, and small
tablets. In addition, hard gelatin capsules can be used for blind-ing in
clinical studies.
The
disadvantages of hard gelatin capsules are owed to the inherent high moisture
content requirement of gelatin. For example, highly soluble salts, such as
iodides, bromides, and chlorides, of drugs are generally not formu-lated in
hard gelatin capsules because these can draw moisture from the shell, thus
making the shell brittle. Storage under low humidity conditions, such as with
the use of desiccant in packaging, can also make the shell brittle. In
addition, gelatin is prone to cross-linking in the presence of very low (in
parts per million range) concentrations of formaldehyde, which may be present
in certain pharmaceutical excipients such as polyethylene glycol (PEG).
Comparison with soft gelatin capsules
In
comparison to soft gelatin capsules, the manufacturing process of hard gelatin
capsules is less demanding, tedious, and costly. This is because the soft
gelatin capsule manufacture requires the formation of gelatin ribbons during
the encapsulation process itself, whereas the hard gelatin capsules use
premanufactured capsule shells. The hard gelatin capsule manufacture also does
not require a curing or moisture-loss step after encapsulation of the drug
formulation.
The
residual water in the capsule shells is lower (~10–16% w/w) for hard gelatin
capsules than for soft gelatin capsules (~30% w/w). This can affect the
stability of the encapsulated formulation directly by chemical degrada-tion (e.g.,
hydrolysis) of water-sensitive compounds or plasticization of the reaction
medium with water, thus increasing the rate of degradation. In addition, soft
gelatin capsule shells have a high oxygen permeation rate, which can contribute
to the oxidation of sensitive drugs.
Solid-filled hard gelatin capsules
Main applications
Hard
gelatin capsules are often preferred over tablets as the dosage form for
initial (Phase I and Phase IIA) clinical studies of new molecular enti-ties
(NMEs). This is because the effect of limited availability of the active
pharmaceutical ingredient (API) to conduct necessary screening for the
development of tablets. Many initial clinical studies simply use a
drug-in-capsule (DIC) product, which is the only drug manually encapsulated in
the hard gelatin or HPMC capsules.
Hard
capsules are also preferred for the comparator and blinded clinical studies.
These clinical studies require that the patient and/or the doctor should not be
able to identify the actual drug product being administered to the patient. In
these studies, two or more drug products are administered after encapsulating
them in hard gelatin capsules of the same specifications and such that the
capsules cannot be opened.
Formulation considerations
Hard
gelatin capsule-manufacturing process places a relatively less strin-gent
requirement on the powder properties of the fill formulation than tab-lets. The
important formulation considerations include the following:
1.
Flow: Adequate flow
through the hopper and into the dosing device (dosator) for reproducible filling of the capsules.
2.
Density: Reproducible
density of the powder is important for fill
weight uniformity of capsules because the dosing devices in high-speed
capsule-filling machines are filled based on the volume of the powder for a
target weight.
3.
Lubricity: Magnesium stearate
is typically added to most powder formulations.
When mixed with other particles, magnesium stea-rate coats their surface and
acts as a lubricant. Lubricants facilitate the lack of adhesion to metallic
machine parts, especially the dosing device used to form a plug in high-speed
machines, and adequate flow of the formulation. Other lubricants commonly used
are stearic acid and sodium stearyl fumarate.
4.
Compactibility: Some high-speed
capsule-filling machines form a plug
of the powder before filling into the capsule. In cases where plug formation is
required for encapsulation, some level of compactibility of the powder is
needed.
5.
Noninteraction with
capsule shell:
Lack of interaction between the drug
substance and/or formulation components with the capsule shell, either gelatin
or HPMC. This interaction could be in the form of solubilization or changing
the water content of the shell. Hygroscopic and volatile components are usually
unsuitable. The fill should not contain more than 5% w/w of water. In addition,
chemical interac-tions between the components can lead to bioavailability or
stability problems. For example, the use of PEG in drug formulation can lead to
cross-linking of gelatin on storage due to the unintended presence of
formaldehyde in PEG, which can diffuse into the shell and react with gelatin.
Similar problems have been observed due to the presence of residual peroxides
in excipients.
6.
Dose: Dose and drug
loading (i.e., %w/w of the formulation,
that is the API) influences drug
content uniformity between the capsules, the extent to which the powder
properties of the formulation are affected by the physicochemical
characteristics of the drug substance, and manufacturability of the capsule
dosage form. For example, it may be difficult to assure adequate uniformity of
the content of the API for drugs with extremely low doses (e.g., in μg), and it may not be possible to
fill a capsule of acceptable size for extremely high-dose drugs (e.g., more
than 600 mg). For intermediate doses, the percent drug loading in the
formulation can range widely. Drug properties predominantly govern the powder
properties of the formulation for high drug-loading formulations (e.g., more
than 60% w/w).
7.
Particle size,
shape, and density: Particle size and shape influence the flow, uniformity, and thus content of the active in a
formulation. A drug substance with irregular or spherical-like crystals is more
likely to flow well than the needle-shaped crystals. Drug content uniformity is
also affected by particle density, if it is significantly different than the
density of the excipients.
8.
Moisture
sorption–desorption isotherm: Moisture sorption and retention properties of the drug and
excipients, indicated by a hys-teresis in the sorption–desorption isotherm, can
affect the physical stability of gelatin during storage and the chemical
stability.
9.
Solubility and wettability: Solubility and
wettability of the drug substance
affect its dissolution characteristics. A low-solubility drug substance might
require the addition of a wetting agent (e.g., surfactant such as polysorbate
80) in the formulation.
Formulation components
The
powder formulations for encapsulation into hard gelatin capsules require a
careful consideration of the filling process requirements, such as lubricity,
compactibility, and flow. Additives present in capsule formula-tions, such as
the amount and choice of fillers, lubricant, disintegrant, and surfactant, and
the degree of plug compaction, can influence drug release from the capsule. The
functional categories of formulation components are as follows:
1. Fillers (or diluents): Active ingredient is mixed
with a sufficient vol-ume of a diluent, usually microcrystalline cellulose,
lactose, man-nitol, starch, or dicalcium phosphate, to increase the bulk of the
formulation.
2. Glidants: Glidants are
finely divided dry powders added to the formu-lation in small quantities to
improve their flow rate from the hopper and into the body of the capsule during
the filling process. Glidants, such as colloidal silicon dioxide, powdered
silica gel, starch, talc, and magnesium stearate, improve flow by
a. Reducing the roughness by filling surface irregularities.
b. Reducing attractive forces.
c. Reducing electrostatic repulsion.
The
optimal concentration of the glidant used to improve the flow of a powder
mixture is generally less than 1% w/w.
3. Lubricants: Capsule formulations
usually require a lubricant just as the
tablet formulations to reduce powder adhesion to the machine parts, especially
during plug formation. Lubricants ease the ejection of plugs by reducing the
adhesion of powder to metal surfaces and fric-tion between the sliding surfaces
in contact with the powder. The most common lubricants for capsule formulations
are hydrophobic stearates, such as magnesium stearate, calcium stearate, and
stearic acid.
4. Surfactants and
wetting agents:
Surfactants may be included in cap-sule formulations of poorly water-soluble
drugs to reduce the contact angle, increase the wettability of drug particles,
and enhance drug dissolution. The most commonly used surfactants in capsule
formula-tions are sodium lauryl sulfate and sodium docusate (sodium dioctyl
sulfosuccinate).
In
addition, a hydrophilic polymer, such as HPMC, is sometimes used as a wetting
agent in the formulations of poorly soluble drugs. Powder wettability and
dissolution rate of several drugs, such as hexo-barbital and phenytoin, were
enhanced with the inclusion of methyl-cellulose or hydroxyethylcellulose in
their capsule formulations.
5. Disintegrants: A disintegrant is
frequently included to aid rapid disin-tegration and dissolution of the
contents. Common disintegrants used in hard gelatin capsule formulations
include croscarmellose sodium, crospovidone, and sodium starch glycolate.
Controlled-release
beads and minitablets are often filled into gelatin capsules for convenient
administration of an oral controlled-release (CR) dosage form. For example, SR
antihistamines, antitussives, and analgesics are first manufactured into
extended-release (XR) micro-capsules or microspheres, and then placed inside a
gelatin capsule. Another example is enteric-coated lipase minitablets that are
placed in a gelatin capsule for more effective protection of these enzymes from
the acidic environment.
Manufacturing process
Very
small-scale and experimental filling of the hard gelatin capsules can simply be
carried out manually, that is, by removing the cap from the body of an empty
capsule shell, filling the body with a preweighed amount of API or formulation,
and attaching the cap. This can be carried out in early clinical studies by the
sponsor or by the pharmacist. Compounding by
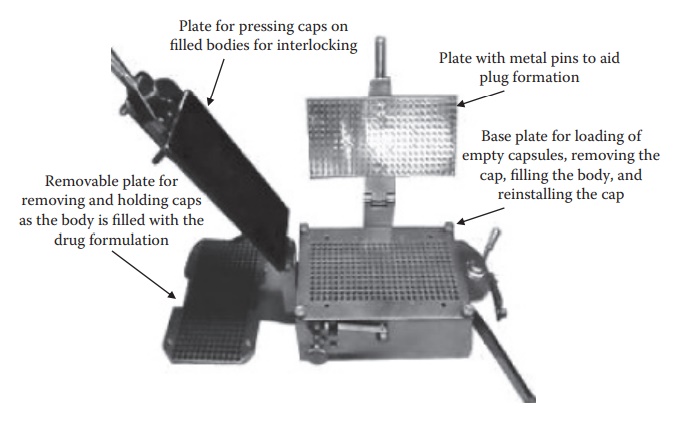
Figure 21.3 Hand-filling machine used to fill hard gelatin capsules.
the
pharmacist is preferred when the stability of the drug in the capsule is
unknown and is called on-site compounding.
Small-scale
manufacture (several hundred capsules) can be done by using a manual
capsule-filling machine. As illustrated in Figure 21.3,
the manual-filling operation involves the following steps:
1. Placing empty gelatin capsules on the removable plate
with bodies facing downward. This removable plate is then placed on the base
plate and the bodies of the capsules are locked in position with the base plate
using a lever.
2. The removable plate is removed with the caps on it. The
body is filled with the formulation manually using a plastic spetula, and the
excess powder is removed.
3. The removable plate is placed back on the base plate and
pressing the flat plate seals the capsule caps. The sealed capsules are removed
from the base plate by opening the lock on the body using the lever and inverting
the base plate.
Large-scale
filling of hard gelatin capsules follows the same principles using a high-speed
capsule-filling machine, with two significant improvements:
·
Capsule alignment and separation are driven by vacuum,
instead of mechanical interlocking.
·
Powder filling may require a soft compact (plug) formation
depend-ing on the formulation weight and capsule fill volume. This compact is
usually much softer than a typical tablet. The compaction force used for plug formation is typically 20–30 N,
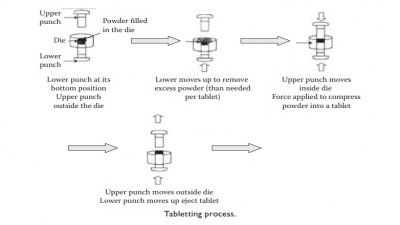
compared to 10–30 kN typically used for tableting.
·
The high-speed powder filling is accomplished by either of
the two dosing devices: (a) dosator device or (b) dosing disk/tamping device.
1. The dosator device
uses an empty tube that dips into powder bed, which is maintained at a height
approximately two-fold greater than the desired length of the plug. The dosator
piston’s forward movement helps form the plug, which is then transferred to the
body of the capsule, and released.
2. The tamping device
operates by filling the cavities bored into the dosing disk, similar to the
die-filling operation during tableting. A tamping punch slightly compresses the
filled powder by repeated action, which is followed by the ejection of the plug
into the capsule body.
Liquid- and semisolid-filled hard gelatin capsules
Main applications
Liquid-
and semisolid-filled hard gelatin capsules are sometimes used to improve the
bioavailability of drug substances with low solubility and wet-tability. Lipids
in the formulation tend to increase the bile flow in vivo and promote drug absorption. For example, mixtures of
mono-, di-, and tri-glycerides of mono- or dicarboxylate esters of PEGs,
commercially available as Gelucire®, are available in various melting point and
hydrophilic–lipophilic balance (HLB) ranges. Oral availability of drug solution
in Gelucire® or in PEG is frequently higher than that of powder drug
formulation. In addition, self-emulsifying and self-microemulsifying drug
delivery systems (SEDDS and SMEDDS, respectively) can significantly improve
drug’s bioavailability, for example, in the case of cyclosporine A and
fenofibrate.
Liquid
filling of hard gelatin capsules may also be indicated in the case of drugs
with extremely low dose (e.g., in μg) and drug loading (e.g., less than
5% w/w) in the formulation to assure uniformity of content. Uniformity of drug
distribution between different dosage units can be higher with a drug solution
in a liquid or semisolid base than a blended powder.
Drugs
with manufacturability issues in a tablet dosage form may also be for-mulated
as a liquid-filled hard gelatin capsules. For example, drugs with low melting
points can show significant sticking issues in both tablet- and powder-filled
capsule dosage forms. Certain drugs with significant instability to light or
moisture can show better stability in liquid or semisolid filled, compared to a
powder-filled, hard gelatin capsule. The presence of an opaque waxy base and a
molecular mixture of the antioxidant with the drug can increase the effectiveness
of environmental protection in the capsule dosage form.
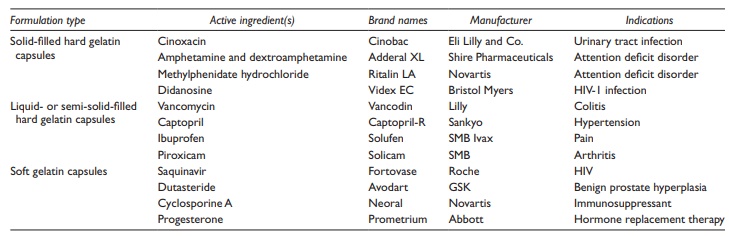
Examples
of drug substances formulated as liquid-filled hard gelatin capsules are listed
in Table 21.2.
Table 21.2 Examples of commonly used capsule dosage forms
Formulation considerations
The
main formulation considerations for liquid-filled hard gelatin capsule are
similar to those for soft gelatin capsules:
1. Noninteraction
with capsule shell: Physicochemical compatibility between the drug/formulation excipients and the capsule shell are
required for any capsule formulation. As described earlier, known drug–gelatin
interactions include pH effect on gelatin hydrolysis or tanning, hygroscopicity
or water effect on shell integrity, and the role of diffusible aldehydes in
cross-linking gelatin shell.
2. Dose: The capsule size
imposes a limit on the maximum amount of formulation
that can be filled into a hard gelatin capsule.
3. Hygroscopicity: The formulation
components should not significantly affect
the moisture level of the shell. For example, highly hygroscopic excipients
such as glycerol, sorbitol, and propylene glycol are not suit-able for
liquid-filled hard gelatin capsules in high concentrations, although they may
be used for soft gelatin capsules. This is because of the lower inherent
moisture content of the hard gelatin shell.
Formulation components
Drug
solution in an appropriate base formulation can be filled into hard gelatin
capsules at room or slightly higher temperature. The functional cat-egories of
formulation components are as follows:
1. Triglycerides for solubilization
of the drug substance. These include either
the medium chain triglycerides, such as Miglyol® 810 and 812, or the long chain
triglycerides, such as soybean oil, olive oil, and corn oil.
2. Surfactants can be included in
the formulation as solubility, dissolu-tion, and/or absorption enhancers, such
as Cremophor®, Gelucire®, Labrafil®, and Tween®.
3. Cosolvents can be used in low
concentrations, especially for SEDDS and
SMEDDS, such as ethanol, propylene glycol, and PEG.
Manufacturing process
The
main consideration and process risk in the manufacture of liquid-filled hard
gelatin capsules is their tendency to leak at the joint between the body and
the cap. This concern has been addressed in one of the two ways:
1.
Applying a zone of gelatin film on the joining region of the body and the cap.
This is known as banding, because a
band of gelatin is formed on the outside of the capsule.
2. Spraying a solution of ethanol and water on the
overlapping areas of the body and the cap along with the application of heat
(e.g., 40°C–60°C for several seconds). This process is known as sealing. The low surface tension of the
solvent mixture allows it to diffuse into and dissolve gelatin, which also
melts during heating, to allow the fusion of gelatin from the cap with that
from the body.
Related Topics