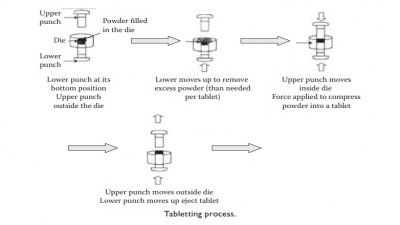
Tablet formulation
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Tablets
In addition to the active drug, called DS or active pharmaceutical ingredi-ent (API), tablets may contain one or more of functional ingredients such as diluents (also known as fillers), binders, disintegrants, glidants, lubri-cants, coating materials, coloring agents, stabilizer(s), sweeteners, and fla-voring agents. These ingredients are called excipients.
Tablet formulation
In
addition to the active drug, called DS or active pharmaceutical ingredi-ent
(API), tablets may contain one or more of functional ingredients such as
diluents (also known as fillers), binders, disintegrants, glidants,
lubri-cants, coating materials, coloring agents, stabilizer(s), sweeteners, and
fla-voring agents. These ingredients are called excipients. Excipients are added to improve one or more of the
three key functional properties of a dosage form: (1) bioavailability, (2)
manufacturability, and (3) stability.
·
Excipients facilitate optimum bioavailability, that is, rate
and extent of drug absorption from the dosage form, by providing reproducible
and optimum rate, extent, and the site of drug release.
·
Excipients facilitate manufacturability by converting the
API powder into a powder blend that flows, compresses, and can be manufactured
on high-speed equipment.
·
Excipients facilitate stability of the API for the duration
of time a product may be stored between the manufacture and the consump-tion
(called shelf life).
Functionality
and examples of these excipient types are listed in Table
20.4.
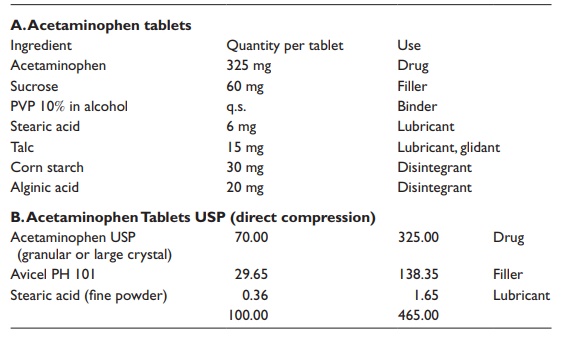
Two
examples of tablet formulations are listed in Table
20.5.
Table 20.4 Functional excipients used in tablets
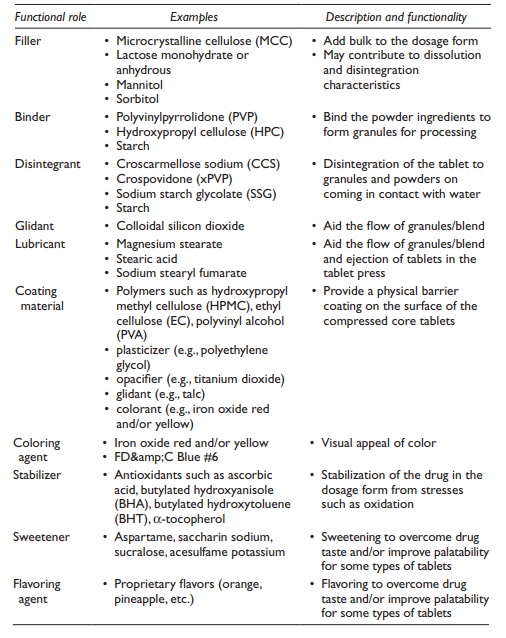
Table 20.5 Examples of immediate release tablet compositions
Diluents
A
tablet should weigh at least about 50 mg for ease of handling by the patient.
Therefore very low-dose drugs invariably require a diluent (also known as
filler) or bulking agent to bring overall tablet weight to at least 50 mg. In
addition, enabling manufacturability of powder blends on high-speed equipment
requires adequate properties such as flow and compress-ibility, which are
improved by the addition of diluents.
Commonly used diluents are microcrystalline cellulose (MCC), lactose, mannitol, sorbitol, and dicalcium phosphate. Each excipient presents characteristics that define its preferred application. For example, lac-tose generally is not preferred for use with drugs that have primary amine groups due to the propensity for Maillard reaction (Figure 20.1).
Mannitol has a negative heat of solution and, thus, provides a cooling sensation in the mouth. It is typically used for chewable and orally dis-solving tablets. MCC can absorb water and improves compressibility by undergoing plastic deformation on compression. Lactose is a fragile excipient that fragments to undergo brittle fracture during compression. Addition of lactose to powder blends can improve interparticle bonding during compression.
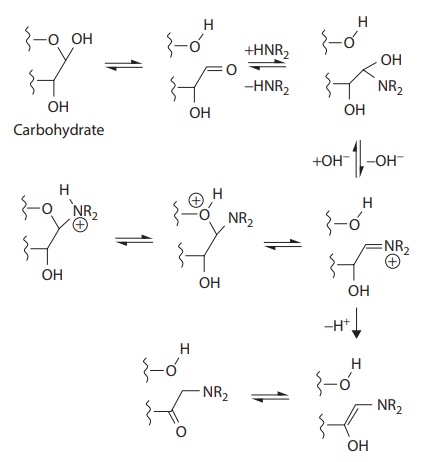
Figure 20.1 Example of Maillard reaction,
followed by Amadori rearrangement, for a secondary amine compound. (From Wirth,
D.D. et al., J. Pharm. Sci., 87(1), 31–39, 1998. With Permission.)
Adsorbents
Adsorbents
are substances capable of holding fluids in an apparently dry state. These are
the powder particles that can adsorb the liquid while main-taining the ability
to be handled as powders. Oil-soluble drugs or fluid extracts can be mixed with
adsorbents to bring them to a solid form for compression into tablets. For
example, fumed silica, microcrystalline cellulose, magnesium carbonate, kaolin,
and bentonite.
Granulating fluid
Granulating
fluids are liquids that are used for wet granulation. Typically, these are
water, ethanol, or isopropanol—or the solution of a hydrophilic polymer
(binder) in one of these liquids. Addition of granulating fluid while mixing a
powder blend of the API with excipients promotes surface adhesion of particles.
After granulation, when the granulating fluid is dried off, the particles tend
to continue to adhere in relatively strong agglom-erates through multiple, weak
noncovalent forces of adhesion among primary particles promoted by the hydrophilic
polymer (binder). These particles tend to have higher sphericity than primary
particles and can be milled or sifted to the desired particle size
distribution. Thus, granulation enables flow and compressibility while
modifying the surface properties of primary particles such as sticking to
stainless steel equipment. The granu-lating fluid is completely removed during
drying to ensure that the residual fluid does not act as a plasticizer and
contribute to chemical instability in the finished DP.
An
alternate process, known as moisture activated dry granulation (MADG), involves
the spray addition of just enough amounts (e.g., 1%–3% w/w) of granulating
fluid, typically water, to dry powder blend to promote surface adhesion during
compression. These processes are typically direct compression processes that do
not require separate granulation and drying unit operations.
Binders
Hydrophilic
polymers are added in either dry or liquid (solution in water) form to promote
the transformation of primary powder particles into cohesive agglomerates
(granules) during wet granulation or to promote cohesive compacts during direct
compression. These hydrophilic polymers are called binders, when used for such
purposes in these formulations. Common binders used in wet granulation are
cellulose derivatives such as hydroxypropyl cellulose (HPC),
polyvinylpyrrolidone (PVP), and starch.
In
wet granulation, the binder can either be dissolved in the granulat-ing fluid
or added dry to the powder mixture followed by the addition of the granulating
fluid for wet granulation. The type and concentration of binder affect the granule strength,
friability, and the granule growth rate during the wet-granulation process.
Size and size distribution of granules can affect the flow and compressibility.
Densification of granules can affect the rate of drug release. For example,
higher molecular weight HPC or PVP binders produce larger and denser granules.
Glidants
Glidants
are added to tablet formulations to improve the flow properties of the granulations
during transfer operations, such as from the hopper to the roller compactor or
tablet press. They improve flow by reducing interpar-ticulate friction. The
commonly used glidants are fumed (colloidal) silica, starch, and talc. These
glidants are very small size powder particles that occupy surface ridges and
irregularities in coarse powder particles, thus increasing the sphericity and
reducing the tendency to adhere to surfaces.
Lubricants
Lubricants
help prevent adherence of the tablet material to the stainless steel processing
equipment surfaces under compression forces. Thus, lubricants reduce or prevent
adhesion of powder particles to tablet compression punch faces and dies or to
the rolls of a roller compactor. They promote flow, reduce interparticle
friction, and facilitate the smooth ejection of com-pressed tablets from the
die cavity. Commonly used lubricants are magne-sium stearate, stearic acid, and
sodium stearyl fumarate. These lubricants are small hydrophobic particles that
tend to coat the surface of larger powder particles by spreading out under the
mild shear during mixing. Hydrophobic surface coating reduces noncovalent
hydrophilic interparticle and particle-equipment forces that are generally
responsible for adhesion and sticking.
Among
these, magnesium stearate is the most commonly used lubricant. The lubricity of
magnesium stearate in a formulation can be increased with either the
concentration or the duration of mixing. Most lubricants are used in
concentration ≤ 1% w/w. Overlubrication, due to the use of high concentration
or excessive mixing can result in reduced compactibility of the blend and/or
rate of drug release from the tablets. Sodium stearyl fuma-rate is the only
water soluble or hydrophilic lubricant and is used in formu-lations that are
highly sensitive to hydrophobic lubricants.
Disintegrants
Disintegrants
are added to the tablets to facilitate the breakup or disinte-gration when
tablets come in contact with aqueous fluids in the GI tract or dissolution
medium during in vitro testing.
Disintegrants help break a com-pressed tablet into constituent granules and
primary powder particles. The dispersed primary API particles then provide a
high surface area for the dissolution of the API in the aqueous fluid. Thus,
breaking of the tablets by disintegrants increases the effective surface area
and promotes rapid release and dissolution of the drug.
Disintegrants
act by either or both (a) swelling in the presence of water and bursting tablet
and granule open and/or (b) capillary action to promote rapid ingress of water
into the center of the tablet or capsule. Examples of commonly used
disintegrants are starch, cross-linked PVP (xPVP), cross-linked sodium carboxymethylcellulose—also
known as croscarmellose sodium (CCS), and sodium starch glycollate (SSG). Among
them, xPVP, CCS, and SSG are called superdisintegrants because these particles
swell significantly in contact with water. Starch swells moderately in contact
with water. Mild disintegrants, such as microcrystalline cellulose (MCC), can
also act by capillary action through their pores to promote the rapid ingress
of water into the center of the tablet.
Both
disintegrants and binders are hydrophilic polymers. For example, PVP is a
binder and xPVP is a disintegrant. Long open chains of hydro-philic polymers
can serve as a binder by promoting hydrogen bonding and hydrophilic bonding
forces on the surface of powder particles. When these polymer chains are cross-linked,
they can imbibe water and swell due to their high hydrogen bonding
capacity—thus acting as a disintegrant. Thus, PVP and xPVP have the same
polymer backbone—while the former is a binder, the latter is a
superdisintegrant.
Miscellaneous
Organoleptic
ingredients, such as colors, sweeteners, and flavors, are used for taste
masking and improving palatability especially in products such as chewable and
dispersible tablets. Artificial sweeteners, such as acesulfame potassium and
saccharin sodium, are preferred because smaller quantities produce similar or
higher sweetness than sucrose.
Chemical
stabilizers commonly used in tablet formulations include anti-oxidants, such as
ascorbic acid and vitamin E, and heavy metal chelators, such as
ethylenediaminetetraacetic acid (EDTA) and ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA).