Complexation and protein binding
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Complexation and protein binding
Complexation is a phenomenon that binds closely one or more molecules of two compounds - a ligand and a substrate by noncovalent attractive forces of interaction.
Physicochemical principles
Complexation and
protein binding
Introduction
Complexation
is a phenomenon that binds closely one or more molecules of two compounds—a
ligand and a substrate by noncovalent attractive forces of interaction. The
resulting structure, in which the ligand is bound to the substrate, is called a
complex. In the case of an administered drug binding a physiological protein,
the drug is the ligand and the protein is called the substrate. A ligand
generally has the ability to complex different types of substrates with similar
binding site—in terms of molecular size, geometry, and charge distribution.
Similarly, a substrate can bind multiple different ligands of similar size,
shape, and surface properties.
In
the case of binding of two small-molecule compounds, either of the two
compounds can be called a ligand or a substrate, depending on the molecular
mechanism of interaction. Thus, a drug molecule can be either a ligand or a
substrate. For example, complex formation of two molecules of theophylline
(substrate) with one molecule of ethylenediamine (ligand) leads to the
formation of the bronchodilator drug aminophylline (Figure
6.1), which has higher solubility than theophylline alone.
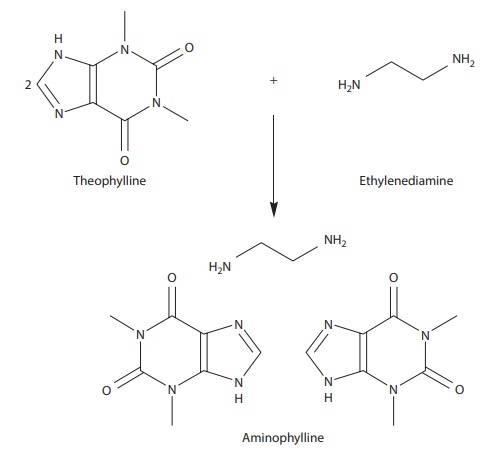
Figure 6.1 Example of complexation. Theophylline and ethylenediamine complex to
yield the bronchodilator drug aminophylline.
On the other hand, aqueous
solubility of oxytetracycline (a drug and a ligand) decreases when it complexes
with calcium ions (substrate) (Figure 6.2),
leading to low oral drug absorption of this antibiotic with dairy products.
Thus, on com-plexation, properties of the drug such as solubility, stability,
partitioning (hydrophilicity/lipophilicity), and absorption are altered.
Plasma–protein
binding (PPP) is usually a reversible interaction of a drug with one or more of
plasma proteins in vivo. The
molecular forces and mechanisms involved in PPP are similar to those in the
complexation phenomenon. Many drugs strongly bind to plasma proteins, such as
albu-min and alpha globulin. Since only the unbound drug is pharmacologically
active and can diffuse out of the bloodstream into various tissue
compart-ments, PPP can influence a drug’s biodistribution (i.e., distribution
inside the body compartments such as the proportion of the drug in the plasma
or the central compartment compared with that in the tissue or the peripheral
compartment), free drug concentration in plasma, and the duration of drug
action. In addition, PPP can lead to drug–drug interactions when two or more
coadministered drugs compete for the same binding site on the protein. For
example, the anticoagulant drug warfarin (Figure 6.2)
is ~97% bound to plasma protein and can be displaced by other highly
protein-bound drugs,
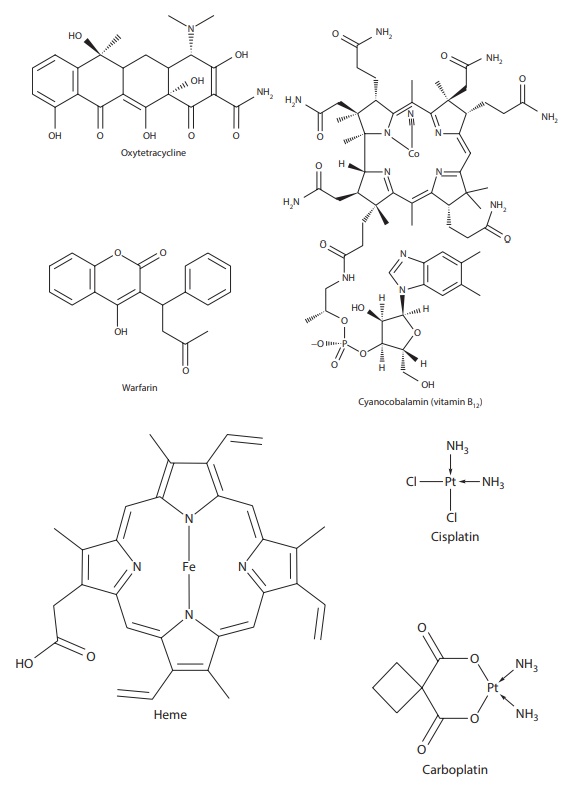
Figure 6.2 Examples of drugs that exist as complexes and/or have a high propensity for forming complexes.
Thus, coadminis-tration of
a drug that displaces warfarin from its protein-binding sites can cause high
free-drug concentration, leading to toxicity of this low therapeu-tic index
drug.
Related Topics
