Concentration calculations
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Pharmacy math and statistics
A formulation is essentially a multicomponent mixture. The relative amount of a substance in a multicomponent system represents its concentration.
Concentration calculations
A
formulation is essentially a multicomponent mixture. The relative amount of a
substance in a multicomponent system represents its concentration. It could be
the concentration of a dissolved drug in a solution, a suspended drug in a
suspension, or a drug powder in a triturate of solid powders. The expression of
concentration, its relation to the total amounts, and calculations involving
changes to the concentration or total amount are an essential part of pharmacy
practice. This section discusses the common ways of expressing concentrations,
their basic principles, and the calcula-tions involving drug amounts in such
preparations.
Percentage solutions
Concentrations
of ingredients in a formula are often represented as a percentage (%).
Percentage represents parts of 100 (cent). In liquid prepara-tions, percentage
values can represent % weight/weight (% w/w, e.g., 2 g of solid in a 100 g of
liquid = 2% w/w), % weight/volume (% w/v, e.g., 2 g of solid in 100 mL of
liquid = 2% w/v), or % volume/volume (% v/v, e.g., 2 mL of liquid A in 100 mL
of liquid B = 2% v/v of liquid A).
Calculations
for the exact amount of an ingredient to be used in a for-mulation when the
percentage composition of the formula is known can be made using ratio and proportion.
Thus, to dispense 240 mL of a 10% w/v solution of a drug substance, the amount
of drug substance needed can be calculated as:
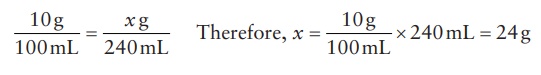
Concentrations based on moles
and equivalents
Molecular
weights or moles of a compound are more useful for calculations when two or
more chemical compounds are to be compared for a given attribute. Thus, during
drug discovery, relative potencies of different com-pounds are compared on a
molar basis.
The
concepts of solution concentrations of compounds are based on their molecular
or equivalent weights, which can be defined as follows:
·
The molecular weight
of a compound represents the weight of one mole (abbreviation: mol) of a
compound, in grams. Thus, 1 mole of glucose is 180.16 g of glucose. The molar
mass of glucose is thus represented as 180.16 g/mol.
·
An equivalent weight
of a compound represents its molecular weight divided by the number of valence
or ionic charges in solution. It takes into account the chemical activity of an
electrolyte. One equivalent (abbreviation: Eq), in grams, of a compound
represents 1 mole of compound in grams divided by its valence. Thus, molecular
weight of Mg2+ ions is 24.3 g, indicating that 24.3 g of Mg2+
ions represents 1 mole of Mg2+. On the other hand, the equivalent
weight of Mg2+ ions is 24.3/2 = 12.15 g, since there are two charges
on Mg2+ ions. Thus, when used for charge-neutralization
calculations, 1 mole or molecular weight of Mg2+ ions represents two
equivalents.
Solutions
of electrolytes are often prepared in terms of molarity, molality, and
normality.
·
Molarity (abbreviation: M) is
defined as the moles of solute per liter of
solution. Therefore, 1 M of sulfuric acid solution represents 98 g (molecular
weight) of H2SO4 dissolved in 1 L of solution.
·
Normality (abbreviation: N)
represents gram equivalent weight of solute
per liter of solution. The difference between molarity and normality is
representative of the difference between moles and equiv-alents of a compound.
Thus, 1 N of sulfuric acid solution represents 49 g (equivalent weight) of H2SO
4 dissolved in 1 L of solution. The equivalent weight of H2SO4
is half its molecular weight, since H2SO4 is a diprotic
acid (i.e., dissociates to release 2 H+ ions in solution).
·
Molality (abbreviation: m) is
a less frequently used term that represents
the number of moles of solute per kilogram of solvent.
·
Formality (abbreviation: F) is
another less frequently used term that represents
the formula weight of a compound in 1 L of solution. It differs from molarity
in indicating the amount of solute added to the solution, but it does not
consider the nature of the chemical species that actually exist in solution.
For example, when 1 mole of sodium carbonate (Na2CO3) or
sodium bicarbonate (NaHCO3) is dissolved in a total of 1 L of an
acidic solution of hydrochloric acid (HCl), the concentration of Na2CO3
or NaHCO3 may be represented as 1 F (indicates the amount added) but
not as 1 M (indicates amount in solution)—even though quantitatively they would
be the same—since the compound reacts with acid in the solution and does not
remain as the same species that was added.
·
The amount of a solute may also be represented as its mole fraction. The mole fraction of a
solute is the number of moles of solute as a proportion of the total number of
moles (of solute + solvent) in a solu-tion. For example, a mole fraction of 0.2
indicates 2 moles of solute dissolved in 8 moles of solvent. Mole fraction is a
dimensionless quan-tity. Mole fraction is frequently used to represent the
relative amount of two different solutes in a system.
Concentrations
and amounts can be represented in fractions by using the prefixes used in the
metric system of measure. Thus, 1 mEq is one milliequivalent of a solute, thus
representing 1/1,000th of an equivalent weight of the solute. Similarly, 1 μM would represent 1 micromolar, or
1/1,000,000th of a molar (1 mol/L) concentration of a solute.
Parts per unit concentrations
Parts
per unit concentrations are commonly expressed for very low con-centrations of
solutes. The commonly used parts per unit concentrations are as follows:
·
Parts per million (ppm) represents 1 part of a substance in
1 million (106) parts of the total mixture. Parts per million (ppm)
is dimension-less, since the parts of both the substance and the total mixture
are represented in the same units. In addition, this measure is applicable to
both solutions and solids. Thus, 1 ppm of NaCl in a solid powder may represent
1 μg/g or 1 mg/kg of
NaCl. Moreover, 1 ppm of NaCl solution can represent 1 μL/L of NaCl in water.
·
Parts per billion (ppb) represents 1 part of a substance in
1 billion (109) parts of the total mixture. Similar to ppm, it is
dimensionless and does not represent a state (solid or liquid) of the
substance.
·
Other less commonly used parts per unit measures are parts
per thousand, parts per trillion (ppt, 1 in 1012), and parts per
quadrillion (ppq, 1 in 1015).
Dilution of stock solutions
A
stock solution is a concentrated solution of a substance that can be diluted to
a lower, desired concentration by adding the solvent immediately before use or
dispensing. Stock solutions are frequently used in pharmacy dispensing to
increase the efficiency, ease, and accuracy of dispensing, as well as the space
and cost advantages with the transportation and storage of lower-volume
concentrated solutions.
A
common calculation required for the dilution of a concentrated stock solution
to a desired concentration is the amount of solvent needed to achieve the
desired concentration. This can be derived from the formula for concentration
(c) based on the volume (v) of solution and the weight (w) of the substance.
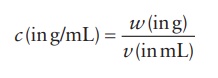
Therefore,
if the stock solution were designated by the subscript “1” and the final
solution to be prepared by the subscript “2,”
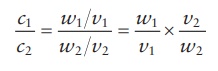
Since
it is the same weight of the solute that would be transferred from the stock
solution into the final, diluted solution,
w1 = w2
Therefore,
c1 × v1 = c 2 × v2 (5.3)
This
formula can be used to calculate the volume of solvent required to make a
diluted solution. For example, to dilute a 50% w/v stock solution to make 200
mL of a 5% w/v solution, c1
= 50, c 2 = 5, and v2 = 200.
v1 = ( c2 × v2 ) / c1 = ( 5 × 200 ) / 50 = 20mL
Hence,
the amount of stock solution needed = 20 mL and the amount of solvent needed =
200 − 20 = 180 mL to make a total of 200 mL of the diluted solution.
The
measurements can also be carried out in weight rather than in vol-ume for the
stock and the diluted solutions. Thus,
c1 × w1 = c 2 × w2 (5.4)
Mixing solutions of different concentrations
Often,
mixing of two products made of the same solute but having different
concentrations is required. A convenient approach to solve these problems is
the alligation method. Two kinds of alligation methods are commonly used:
alligation medial and alligation alternate.
Alligation medial
For two ingredients
This
method is based on finding the proportion for a formula. The strength (e.g., %
w/w) of an ingredient is multiplied by its amount (e.g., quantity in grams) to
obtain the product of each ingredient. The products of all ingre-dients and
their quantities in the original formula are added together sepa-rately.
Dividing the sum of products by the sum of quantities in the original formula
gives a quotient, which represents the strength (e.g., % w/w) of the final
mixture.
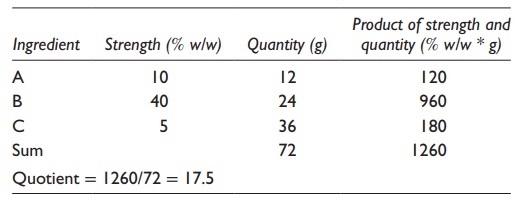
For
example, to calculate the strength of the final mixture when 12 g of a 10% w/v
sucrose solution is mixed with 24 g of a 40% w/v sucrose solution, one would
write the alligation medial method as indicated in Table
5.1. Working through this table, the final solution would be of 30.0%
w/w strength.
For more than two ingredients
This
method is also applicable for more than two ingredients. For example, to
calculate the strength of the final mixture when 12 g of a 10% w/v sucrose
Table 5.1 Alligation medial method for
two ingredients
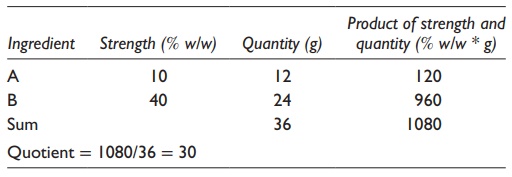
Table 5.2 Alligation medial method for
more than two ingredients
Alligation alternate
For two ingredients
This
method can be used to calculate the amount of a diluent, solute, or
different-concentration product that would need to be added to a given
concentration product to make a new concentration preparation. The num-ber of
parts required for the lower- and higher-concentration preparations to make the
target-concentration preparation is obtained by constructing a matrix and doing
the calculation as shown in Table 5.3.
Table 5.3 Alligation alternate method for
two ingredients
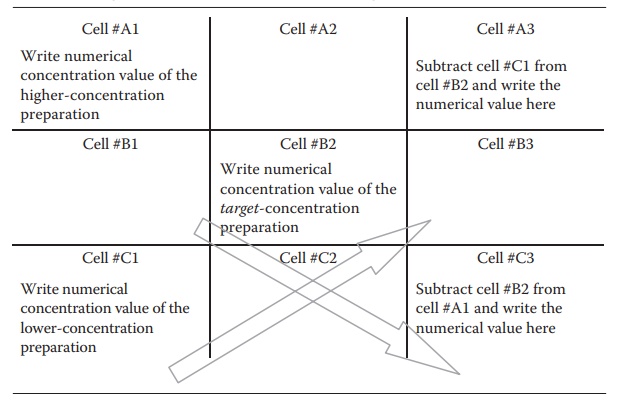
Thus, subtracting the target concentration from the lower concentration gives the target amount of the higher-concentration preparation, and subtracting
Thus, the total amount of the
target-concentration preparation that would be prepared can be obtained by
adding together the target amounts of higher- and lower-concentration
preparations needed. If the required amount of the target-concentration
preparation is dif-ferent than the amount obtained by the formula, the
principles of propor-tion, discussed earlier, can be used to calculate the
quantities needed for the required total amount of the target-concentration
preparation.
For
example, to prepare 200 mL of a 12% w/v sucrose solution using a 40% w/v and
another 5% w/v sucrose solution, one would write the alligation matrix as shown
in Table 5.4.
Thus,
combining 7 mL of 40% w/v solution with 28 mL of 5% w/v solution would give 7 +
28 = 35 mL of 12% w/v solution. To make 200 mL of 12% w/v solution, one would
use the principles of proportion as follows:
For
the quantity of 40% w/v solution,
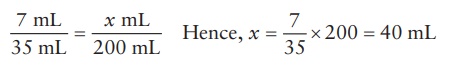
For
the quantity of 5% w/v solution,
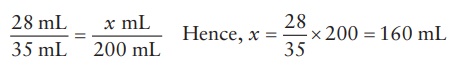
Alternatively,
a conversion factor could be derived for the calculation:
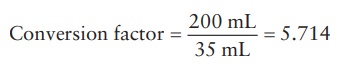
The required quantities of low- and high-concentration solutions can then simply be obtained by multiplying their quantities obtained by the alliga-tion formula by this factor. Thus, the quantity of 40% w/v solution required = 7 × 5.714 = 39.998 = 40 mL. Therefore, the quantity of the 5% w/v solution required = 200 − 40 = 160 mL or 28 × 5.714 = 159.992 = 160 mL.
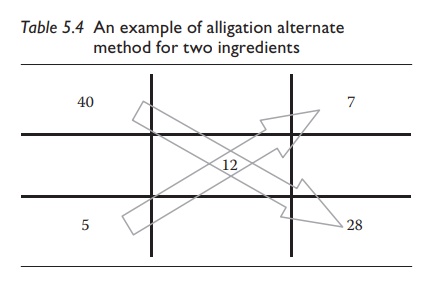
Table 5.5 An example of alligation
alternate method for more than two ingredients
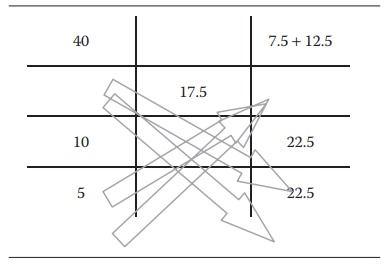
For more than two ingredients
The
alligation alternate method can be used for more than two ingredients by pairing off the values of one higher
(than the desired) strength ingredient with two lower (than the desired)
strength ingredients, or vice versa. This is illustrated by the following
example:
To
prepare a 17.5% w/w solution using a 10% w/v, a 40% w/v, and a 5% w/v sucrose
solution, one would write the alligation alternate method, as shown in Table 5.5.
Thus,
combining 20 mL of 40% w/v solution with 22.5 mL of 10% w/v solution and 22.5
mL of a 5% w/w solution would give 20 + 22.5 + 22.5 = 65 mL of 17.5% w/v
solution. The laws of proportion can be used, as described earlier, to
calculate specific quantities of starting solutions that would be needed to
prepare a desired quantity of the final solution.
The
alligation alternate method for more than two ingredients can use any pairing
of higher (than the desired) strength ingredient(s) with lower (than the
desired) strength ingredient(s). The pairings can be any number depending on
the number of ingredients.
The
alligation methods are applicable to all forms of preparations, including
powders. In addition, the alligation method can also be used for calculating
the required quantities for dilution of a preparation with the solvent or
diluent alone by making the concentration of the lower-concentration
preparation zero.
Tonicity, osmolarity, and preparation of isotonic solutions
Of
the two compartments of solution separated by a semipermeable membrane, the
solvent tends to flow from the solution with the lower solute concentration to
the solution with the higher solute concentration. If uninterrupted flow of
solvent is allowed, it would result in the equaliza-tion of concentration
across the membrane. This phenomenon is called osmosis. The pressure of solvent involved in this phenomenon is
termed osmotic pressure. A solution containing a nonpermeable solute
creates a pressure for the inward
flow of solvent across the semipermeable mem-brane. Thus, osmotic pressure can
also be defined as the pressure that must be applied to a solution to prevent
the inward flow of solvent across a semipermeable membrane.
Tonicity
is the osmotic pressure of two solutions separated by a semiper-meable
membrane. Tonicities of solutions are often represented with refer-ence to that
of normal body fluids. Thus, solutions that exert lower osmotic pressure than
the body fluids are termed hypotonic,
while solutions that exert higher osmotic pressure than the body fluids are
termed hypertonic. Hypotonic
solutions have lower and hypertonic solutions have higher impermeable solute
concentration than the body fluids. Two solutions that have the same osmotic
pressure are termed isosmotic, while
a solution that has the same osmotic pressure as a reference body fluid is
termed isotonic.
To
define the osmotic amount and concentration of a solute without referring to
another solution, the concepts of osmole, osmolarity, and osmolality are
introduced. An osmole is the amount
of a substance that represents the number of moles of particles that it forms
in a solution. For a nondissociating substance, that is, a nonelectrolyte such
as dextrose, 1 osmole = 1 mole. Thus, 1 osmole of dextrose = 186 g (molecular
weight) of dextrose.
Similar
to the concept of molarity, osmolarity
(abbreviation: Osmol) is defined as the osmoles of solute per liter of
solution. Therefore, 1 Osmol of glucose solution represents 186 g (molecular
weight) of glucose dissolved in 1 L of solution.Similar to the concept of
molality, osmolality is defined as
the osmoles of solute per kg of solvent. These quantities can be used with
prefixes in the metric system, such as milli and micro. Thus, a commonly used
term is milliosmole (abbreviation: mOsmol), which represents 1/1,000th of an
Osmol. Moreover, while osmole represents the quantity of solute in grams, Osmol
represents the concentration of solute in a solution.
For
a dissociating solute, such as an electrolyte, 1 mole ≠ 1 Osmol and 1 M
solution ≠ 1 Osmol solution. The osmoles and osmolarity of such a solute are
calculated by multiplying with the number of particles formed on dissociation
and the fractional degree of dissociation of a substance in solution. Thus,
assuming complete dissociation, NaCl, CaCl2, and FeCl3
form 2, 3, and 4 particles in solution. Thus, 1 mM solution of NaCl, CaCl2,
or FeCl3 represents their 2, 3, or 4 mOsmol solution, respectively.
Assuming, 80% degree of dissociation for dilute solutions, 2 M of NaCl, CaCl2,
and FeCl3 solutions represent:
2
× (1 + 80/100) = 3.6 Osmol of NaCl solution
2
× (1 + 80/100 + 80/100 ) = 5.2 Osmol of CaCl2 solution
2
× (1 + 80/100 + 80/100 + 80/100 ) = 6.8 Osmol of FeCl3
solution
The
normal serum osmolality is in the range of 275–300 mOsmol/kg. osmolality of
solutions can be measured in the laboratory by using an osmometer.
Tonicity
is an important concept in the administration of ophthalmic and parenteral
solutions. Hypertonic solutions tend to draw fluids out of body tissues,
leading to irritation and dehydration. Hypotonic solu-tions, on the other hand,
can provide excess fluid to the body tissues. However, since the volume of the
administered solution is much lower than that of body fluids and fluid elimination
is a regulated physiological phenomenon, hypotonic solutions are relatively
inconsequential. Thus, administration of hypertonic solutions tends to be more
tissue-damaging and painful than the administration of hypotonic solutions.
Nonetheless, isotonic solutions are better tolerated by patients than either
extremes of tonicity.
Preparation
of isotonic solutions requires the use of one of the col-ligative properties of
solutions. Colligative properties are the solution properties that depend on the
number of molecules of solvent in a given volume of solution but are
independent of the properties of the solute. These properties include lowering
of vapor pressure, elevation of boiling point, osmotic pressure, and depression
of freezing point of a solution with increasing solute concentration. Of these,
the depression of freezing point is conveniently used to calculate the amount
of solute required to prepare an isotonic solution.
For
example, given that the freezing point of blood serum and ophthalmic lachrymal
fluid is −0.52°C and that 1 M aqueous solution of a nonelectro-lyte depresses
the freezing point of water by 1.86°C, we can calculate the amount of glucose
(molecular weight: 180 g/mol) required to prepare an isotonic solution by
solving for the amount of glucose that would produce a freezing point
depression of 0.52°C. Thus, to make 1 L of isotonic glucose solution, the
amount of glucose required (x) can be
calculated as:

This
corresponds to 5% w/v glucose solution. The commonly available dextrose
solution for intravenous (IV) administration has this concentra-tion. Similar
concentration for an electrolyte, such as sodium chloride, should take into
consideration the dissociation constant of the solute and the number of species
produced in the solution. Thus, assuming that NaCl in weak solutions is about
80% dissociated, the total number of solutes in solution would be 1.8 times the
number of molecules added to the solution. This (1.8) dissociation factor (abbreviation: i) is used in the calculation of isotonic concentrations of
electrolytes. Thus, to make a 1 L isotonic NaCl (molecular weight: 58 g/m)
solution, the amount of NaCl required (x)
can be calculated as:
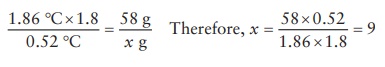
This
corresponds to 0.9% w/v NaCl solution, which is commonly available as an
isotonic solution for experiments involving living cell and tissues. From these
calculations, note that 50 g/L of glucose solution is isotonic to 9 g/L of NaCl solution. Therefore, in quantities of
solutes, 50 g of glucose is tonic
equivalent to 9 g of NaCl. The tonic equivalence of two substances
represents their amounts that would produce the same osmotic pressure. Thus,
the quantity of any substance divided by its dissociation factor, i, represents its tonic equivalent
quantity to any other substance. This principle is used in the preparation of
isotonic solutions by the addi-tion of NaCl to hypotonic drug solutions to
increase the tonicity to the physiological equivalent of 0.9% w/v NaCl. Using
the above conversion of tonic equivalents, NaCl
equivalents (E values) of various
substances are known in the literature. The number of grams of all ingredients
in a pre-scription is multiplied by their E
values and added together to determine the osmotic equivalent of NaCl amount
represented by the substances. In addition, the amount of NaCl that would be
required to make a 0.9% w/v solution of the same volume as the prescription is
determined. Subtracting the former from the latter gives the amount of NaCl
needed to make the solution isotonic. Any substance other than NaCl, such as
dextrose, can also be used to increase the tonicity of a solution by dividing
the amount of NaCl needed by the NaCl equivalent of the other substance.
For
example, to compound 10 mL of an ophthalmic preparation of 3% w/v pilocarpine
nitrate, we first determine the amount of drug in 10 mL of solution.
Drug
amount = 3/100 ×10 = 0 .3 g
The
NaCl equivalent (E value) of
pilocarpine nitrate (molecular weight 271, dissociates into 2 ions, and i value = 1.8) can be read from the
literature or calculated as:
E
value = (58. 5 / 1.8) /
(271 / 1.8) = 0.216
Now,
we multiply the E value with the drug
amount in solution to get NaCl equivalents represented by the drug amount in
the solution:
NaCl
equivalent in prescription =
0.3× 0.216 = 0.0648 g
This
is the amount of particles in solution equivalent to NaCl, which must be
subtracted from the amount of NaCl that would be needed to make an isotonic
solution of the same volume as the prescription (i.e., 10 mL). This is
calculated as:
Total
amount of NaCl needed for isotonicity = (0.9/100) × 10 = 0 .09 g
Hence,
the amount of NaCl that must be added to the prescription to make an isotonic
solution = 0.09−0.0648 = 0.0252 g.
If
a prescription contains multiple components, NaCl equivalent for each component
is calculated separately and added together to make the total NaCl equivalents
in the prescription. This total amount is then subtracted from the total NaCl
that would be needed for isotonicity of the volume of pre-scription to obtain
the amount of NaCl that must be added to the prescription.
Related Topics
