Conjugation With Glucuronic Acid
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Biotransformation of Drugs
Also called as glucuronidation, it is the most common and most important phase II reaction.
Phase II reaction
CONJUGATION WITH GLUCURONIC ACID
Also called as glucuronidation,
it is the most common and most important phase II reaction for several reasons:
1.
Readily available source of
conjugating moiety, D-glucuronic acid which is derived from D-glucose.
2.
Several functional groups viz. alcohols, acids, amines, etc. can
combine easily with D-glucuronic acid.
3.
Quantitatively, conjugation with
D-glucuronic acid occurs to a high degree.
4.
All mammals have the common
ability to produce glucuronides,
5.
The free carboxyl function of
glucuronic acid has a pKa in the range 3.5 to 4.0 and hence
ionisable at both plasma and urine pH thereby greatly increasing the water
solubility of the conjugated substrate.
6.
The glucuronidation enzymes are
in close association with the microsomal mixed function oxidases, the major
phase I drug metabolising enzyme system; thus, a rapid conjugation of phase I
metabolites is possible.
7.
Lastly, glucuronidation can take
place in most body tissues since the glucuronic acid donor, UDPGA is produced
in processes related to glycogen synthesis and thus, will never be deficient
unlike those involved in other phase II reactions.
Glucuronide formation occurs in 2 steps –
1. Synthesis of an activated coenzyme
uridine-5'-diphospho-α -D-glucuronic acid (UDPGA) from UDP-glucose (UDPG). The
coenzyme UDPGA acts as the donor of glucuronic acid. UDPG is synthesized by
interaction of α-D-glucose-1-phosphate with uridine triphosphate (UTP).
2. Transfer
of the glucuronyl moiety from UDPGA to the substrate RXH in presence of enzyme UDP-glucuronyl
transferase to form the conjugate. In this step, the - configuration of
glucuronic acid undergoes inversion
and thus, the resulting product is – β-D-glucuronide (also called as glucosiduronic acid or glucopyranosiduronic acid conjugate).
The steps involved in glucuronide synthesis are
depicted below:
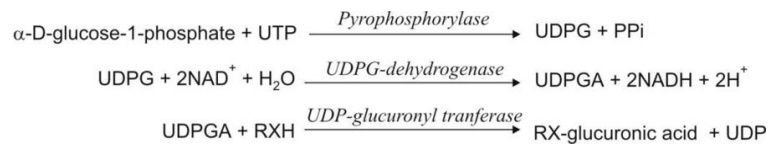
where X = O, COO, NH or S.
An example of glucuronidation of benzoic acid is
shown below.
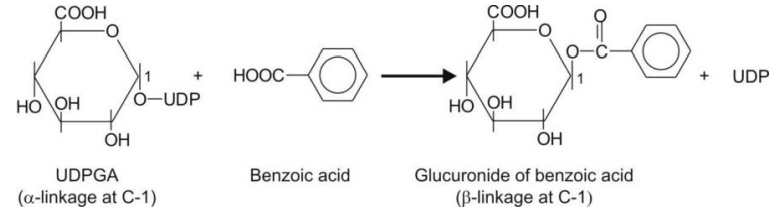
A large number of functional groups are capable of
forming oxygen, nitrogen and sulphur glucuronides. Carbon glucuronides have
also been detected in a few cases.
Oxygen or O-Glucuronides
Xenobiotics with hydroxyl and/or carboxyl functions
form O-glucuronides.
1. Hydroxyl Compounds: These
form ether glucuronides. Several examples of such compounds are given below.
Aliphatic alcohols e.g. chloramphenicol,
trichloroethanol
Alicyclic alcohols e.g. hydroxylated hexobarbital
Arenols (phenols) e.g. morphine, paracetamol
Benzylic alcohols e.g. methyl phenyl carbinol
Enols e.g. 4-hydroxy coumarin
N-hydroxyl amines e.g. N-hydroxy dapsone
N-hydroxyl amides e.g. N-hydroxy-2-acetyl
aminofluorine
2. Carboxyl Compounds: These form ester
glucuronides
Aryl acids e.g. salicylic acid
Arylalkyl acids e.g. fenoprofen
Nitrogen or N-Glucuronides
Xenobiotics with amine, amide and sulphonamide
functions form N-glucuronides.
Aliphatic 2o amines e.g.
desipramine
Aliphatic 3o amines e.g.
tripelennamine
Nonaromatic 3o heterocyclic
amines e.g. cyproheptadiene
Amides e.g. meprobamate
Sulphonamides e.g. sulphadimethoxine
Sulphur or S-Glucuronides
Thiols (SH) form thioether glucuronides e.g. thiophenol.
Carbon or C-Glucuronides
Xenobiotics with nucleophilic carbon atoms such as
phenylbutazone form C-glucuronides.
Certain endogenous compounds such as steroids,
bilirubin, catechols and thyroxine also form glucuronides.
Related Topics
