Reductive Reactions
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Biotransformation of Drugs
Bioreductions are also capable of generating polar functional groups such as hydroxy and amino which can undergo further biotransformation or conjugation.
PHASE I REACTIONS
REDUCTIVE REACTIONS
Bioreductions are also capable of generating polar
functional groups such as hydroxy and amino which can undergo further
biotransformation or conjugation. A number of reductive reactions are exact
opposite of oxidation. For example:
Alcohol dehydrogenation ↔ Carbonyl reduction
N-Oxidation ↔ Amine
oxide reduction
Thus, in this sense, bioreduction comprises one-half of reversible reactions. Such
reactions may be catalysed by –
·
Same enzyme (true reversible reaction), or
·
Different enzymes (apparent reversible reaction).
Since reversible reactions usually lead to
conversion of inactive metabolites into active drug, they may result in delay
of drug removal from the body and hence prolongation of action.
Reduction of Carbonyls (Aldehydes and Ketones)
Depending on their reactivity towards bioreduction,
carbonyls can be divided into 3 categories –
1. The aliphatic aldehydes and
ketones.
2. The aromatic aldehydes and ketones.
3. The esters, acids and amides.
The order of reactivity of these categories of
drugs in undergoing reduction is –
1>2>3
i.e. aliphatic aldehydes and ketones undergo
extensive bioreduction whereas esters, acids and amides are least reactive.
Few aldehydes undergo reduction because such a
reaction is usually reversible, and secondly, they are susceptible towards
oxidation which yields more polar products. Several ketones undergo reduction
as it results in more polar metabolites. Reduction of aldehydes and ketones
yields primary and secondary alcohols respectively. The reaction is catalysed
by non-microsomal enzymes called as aldo-keto-reductases.
A representative example of compounds undergoing
reductive reactions is given below.
Aliphatic aldehydes e.g. chloral hydrate.

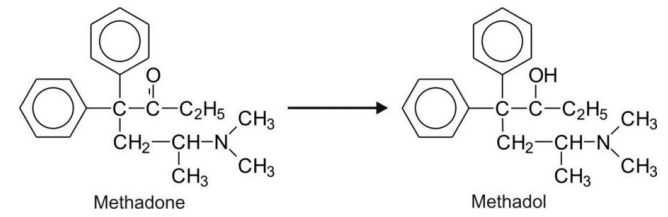
Aliphatic ketones e.g. methadone.
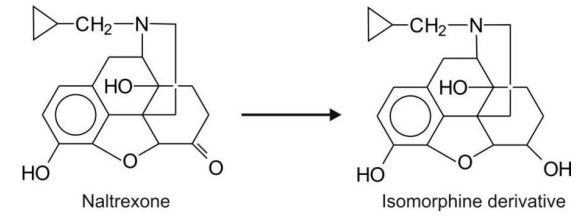
Alicyclic ketones e.g. naltrexone.
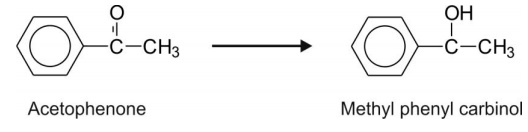
Aromatic ketones e.g. acetophenone.
Reduction of Alcohols and Carbon-Carbon Double Bonds
These two reductions are considered together because
the groups are interconvertible by simple addition or loss of a water molecule.
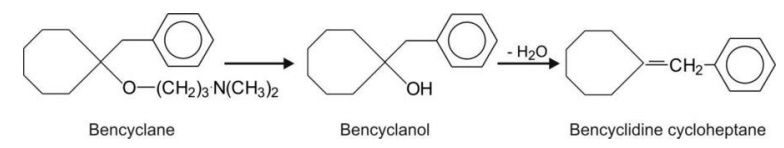
Before an alcohol is reduced, it is dehydrated to C=C bond, e.g. bencyclane
(antispasmodic).
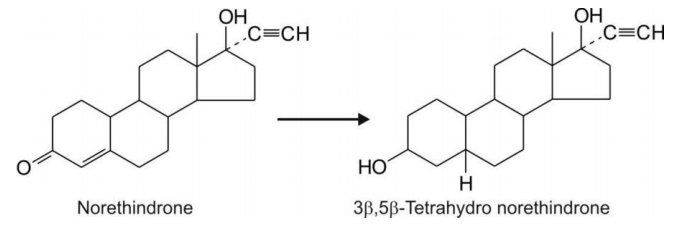
Reduction of norethindrone, an , -unsaturated carbonyl
compound, results in both reduction of C=C double bond and formation of
alcohol.
Reduction of N-compounds (Nitro, Azo and N-Oxide)
The N-containing functional groups that commonly
undergo bioreduction are nitro, azo and N-oxide. It is important to note that
such a reaction is reverse of oxidation.
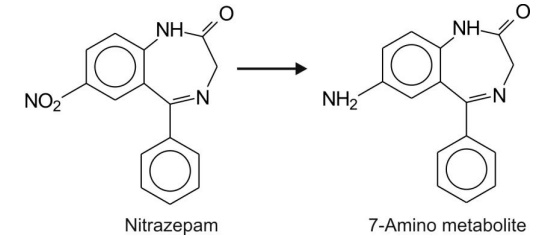
Reduction of nitro group proceeds via formation of nitroso and
hydroxylamine intermediates to yield amines.

For example, reduction of nitrazepam.
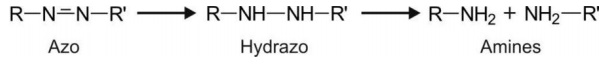
Reduction of azo compounds yields primary amines via formation of hydrazo intermediate
(-NH-NH-) which undergoes cleavage at N-N bond.
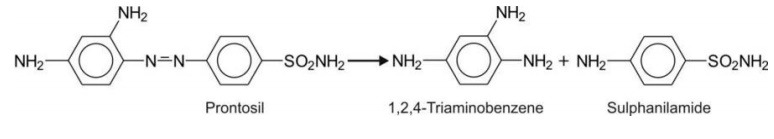
An important example of azo reduction is prontosil.
It is reduced to the active form – sulphanilamide.
Aliphatic and aromatic tertiary amine N-oxides are
reduced to the corresponding amines, e.g. imipramine N-oxide to imipramine.
Miscellaneous Reductive Reactions
1. Reductive Dehalogenation: This
reaction involves replacement of halogen attached to the carbon with the H-atom, e.g. halothane.
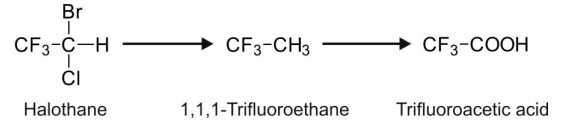
The C-F bond is resistant to reduction.
2. Reduction of Sulphur Containing Functional Groups: An example of S-S reductive cleavage
is disulphiram.
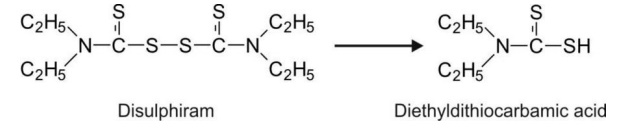
Sulphoxides are sometimes reduced to sulphides,
e.g. sulindac.
Related Topics
