Phase II Reactions
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Biotransformation of Drugs
Phase II reactions are the real drug detoxication pathways
PHASE II REACTIONS
Phase II reactions involve transfer of a suitable
endogenous moiety such as glucuronic acid, sulphate, glycine, etc. in presence
of enzyme transferase to drugs or metabolites of phase I reactions having
suitable functional groups to form highly polar, readily excretable and
pharmacologically inert conjugates.
Phase II reactions are the real
drug detoxication pathways because –
1. The conjugates/products of
phase II reactions are absolutely free of pharmacological activity.
2. The conjugates/products of
phase II reactions are highly polar and thus easily excretable either in bile
or urine.
3. Tissue-reactive and
carcinogenic metabolites formed as a result of phase I reaction are rendered
harmless by conjugation with moieties such as glutathione.
The moieties
transferred to the substrates (called as conjugating reagents) in
a phase II reaction possess 3 characteristics:
1. They are simple endogenous
molecules such as carbohydrates, proteins and fats.
2. They are of large molecular
size.
3. They are strongly polar or
ionic in nature in order to render the substrate water-soluble.
Two outstanding
characteristics of conjugation reactions are –
1. The reaction involves an
initial activation step – either
(a) The drug is activated e.g.
conjugation with amino acids and acetylation reaction; or
(b) The conjugating reagent is
activated e.g. glucuronidation, sulphation and methylation.
2. The reaction is
capacity-limited – the limited capacity of conjugation reactions is attributed
to –
(a) Limited amount of conjugating
agent, for example, glycine.
(b) Limited ability to synthesise
the active nucleotide intermediate.
(c) Limited amount of enzyme
conjugate transferase.
Thus, when doses of drugs are higher than normal
levels of conjugating molecules, saturation of metabolism occurs and the
unconjugated drug/metabolite precipitates toxicity. The order of capacities of
important conjugation reactions is –
Glucuronidation > Amino Acid
Conjugation > Sulphation and Glutathione Conjugation
The increase in the molecular weight of the drug
following conjugation with glucuronic acid, sulphate and glutathione is 176, 80
and 300 Daltons respectively.
The molecular weight of the conjugate is important
in dictating its route of excretion –
·
High molecular weight conjugates (>350)
are excreted predominantly in bile
·
Low molecular weight conjugates
(<250) are excreted in urine.
Thus, glutathione conjugates are always excreted in
bile.
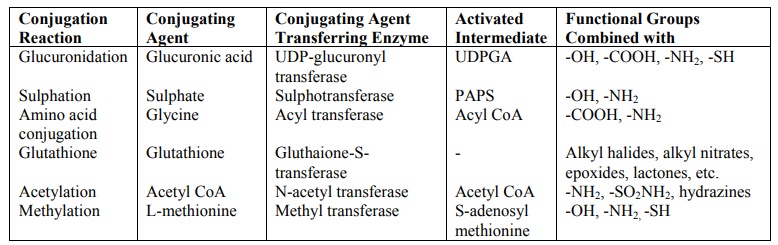
Table 5.3 compares the various phase II reactions.
TABLE 5.3
Phase II Reactions and their Characteristics
Related Topics
