Conjugation With Sulphate Moieties
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Biotransformation of Drugs
Sulphation is similar to glucuronidation but it is catalysed by nonmicrosomal enzymes and occurs less commonly as the moiety that transfers sulphate to the substrate is easily depleted.
Phase II reaction
CONJUGATION WITH SULPHATE MOIETIES
Sulphation is similar to glucuronidation but
it is catalysed by nonmicrosomal enzymes and occurs less commonly as the moiety that transfers sulphate to the
substrate is easily depleted. This process is thus, easily saturable in
comparison to glucuronidation.
·
Sulphation is dominant at low
substrate concentration, whereas
·
Glucuronidation is dominant at
high substrate concentration.
Like glucuronidation, sulphation also occurs in 2
steps:
1. Synthesis of an activated coenzyme
3'-phosphoadenosine-5'- phosphosulphate (PAPS) which acts as a donor of sulphate to the substrate. This also
occurs in two steps —
(a) An initial interaction
between the sulphate and the adenosine triphosphate (ATP) to yield
adenosine-5'-phosphosulphate (APS), followed by
(b) Activation of APS to PAPS.
2. Transfer of sulphate group from PAPS to
the substrate RXH in presence of enzyme sulphotransferase
(sulphokinase) and subsequent liberation of 3'-phosphoadenosine-5'-phosphate
(PAP).
The steps are summarized in the equations below:
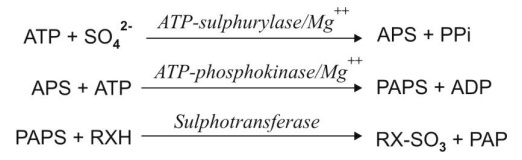
where X = O, NH
Functional groups capable of forming sulphate
conjugates include phenols, alcohols, arylamines, N-hydroxylamines and
N-hydroxyamides. The reaction product is a sulphate ester, also called as ethereal sulphate.
Examples of compounds undergoing sulphation are:
Phenols e.g. paracetamol, salbutamol
Alcohols e.g. aliphatic alcohols C-1 to C-5
Arylamines e.g. aniline.
Sulphoconjugates can be tissue reactive, e.g. the
O-sulphate conjugate of N-hydroxy phenacetin covalently binds to hepatic and
renal tissues.
Endogenous substances can also undergo sulphation,
e.g. steroids, biologic amines, etc.
Related Topics
