Biotransformation of Drugs
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Biotransformation of Drugs
Biotransformation of drugs is defined as the chemical conversion of one form to another. The term is used synonymously with metabolism.
Biotransformation
of Drugs
The onset of
pharmacological response depends upon two pharmacokinetic processes—
·
Drug absorption, and
·
Drug distribution (since most
sites of action are in the extravascular tissues).
The duration
and intensity of action depend upon –
·
Tissue redistribution of drug,
and
·
The rate of drug removal from the
body/site of action, i.e. rate of elimination.
Elimination is the major process for removal
of a drug from the body and termination of
its action. It is defined as the
irreversible loss of drug from the body. Elimination occurs by two
processes viz. biotransformation and
excretion.
Biotransformation of drugs is defined as the chemical conversion of one form to another. The term is used
synonymously with metabolism. The
chemical changes are usually affected enzymatically in the body and thus, the
definition excludes chemical instability of a drug within the body; for e.g.
conversion of penicillin to penicilloic acid by the bacterial penicillinase and
mammalian enzymes is metabolism but its degradation by the stomach acid to penicillenic
acid is chemical instability.
Need for Drug Biotransformation
All chemical substances that are not nutrients for the body and enter the body through, ingestion, inhalation or absorption are called as xenobiotics (Greek: xenos = foreign) or exogenous compounds. Drugs are also xenobiotics which enter the body by virtue of their lipophilicity. It is interesting to note that for effective absorption, a drug needs to be sufficiently lipid soluble but it is this same physicochemical property that enables it to bypass excretion. This is because only water-soluble agents undergo renal excretion (major route for exit of drugs from the body) whereas lipid soluble substances are passively reabsorbed from the renal tubules into the blood after glomerular filtration. Thus, if such a phenomenon continues, drugs would accumulate in the body and precipitate toxic reactions. However, to prevent such a consequence, the body is armed with the metabolic system which transforms the water insoluble, lipophilic, nonpolar drugs into polar and water-soluble products that can be easily excreted by the kidneys and are poorly reabsorbed; for instance, hippuric acid, the metabolite of benzoic acid, is 2.5 times more water-soluble. Drug biotransformation is thus a detoxification process. However, exceptions are there when biotransformation leads to products with decreased water solubility. The N-acetyl derivatives of sulphonamides are less water-soluble than the parent drug and thus have a tendency to cause crystalluria. Figure 5.1 illustrates the disposition of drug in the body as a result of metabolism.
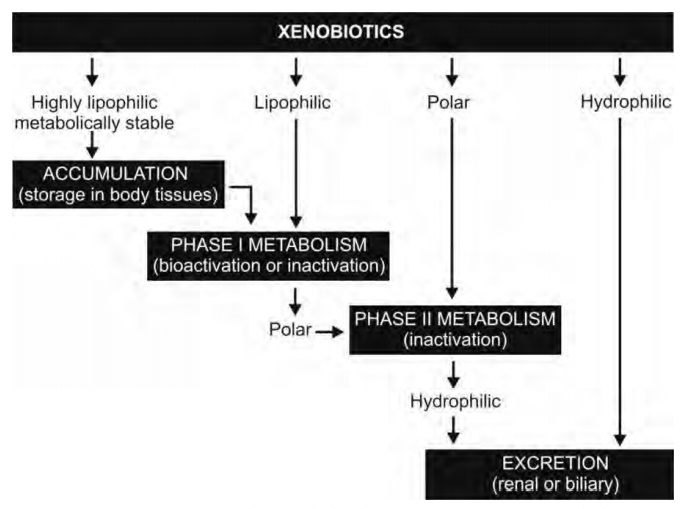
Fig. 5.1. Disposition of drug in the body
as a consequence of metabolism
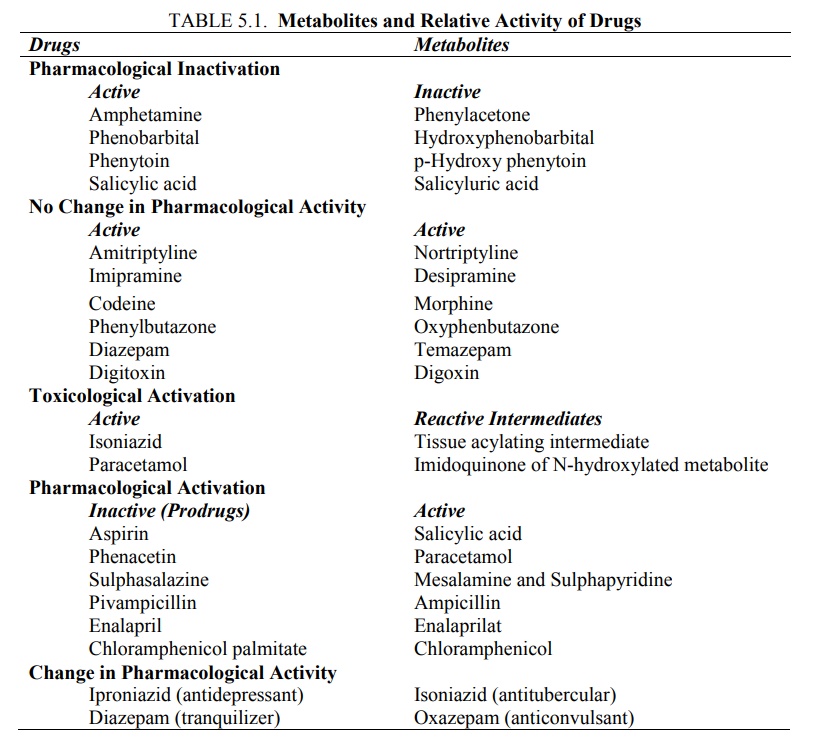
Biotransformation –
·
Normally results in pharmacological
inactivation of drugs, i.e. it
results in formation of metabolites with little or no pharmacological activity;
e.g. conversion of phenytoin to p-hydroxy phenytoin.
·
Occasionally yields metabolites with equal activity; e.g. conversion of
phenylbutazone to oxyphenbutazone.
·
Rarely leads to toxicological activation of drugs, i.e. it results in
formation of metabolites with high tissue reactivity; e.g. conversion of
paracetamol to reactive metabolites that cause hepatic necrosis.
Inactive drugs (prodrugs) also depend upon
biotransformation for activation, the process being called as pharmacological activation; e.g.
conversion of enalapril to enalaprilat. A change in pharmacological activity of
the drug on metabolism has also been observed (see Table 5.1.).
TABLE 5.1. Metabolites
and Relative Activity of Drugs
In comparison with xenobiotics, the natural
endogenous substances such as neurotransmitters (dopamine, GABA, epinephrine,
norepinephrine, etc.), steroids (testosterone, progesterone, cortisol, etc.)
and insulin which are also used as therapeutic agents, are inactivated rapidly
because of the body’s well developed system for metabolising such agents. These
substances are therefore called as soft
drugs. Such soft drugs do not precipitate unexpected toxicity when used in
concentrations close to their normal levels.
Drug Metabolising Organs
Liver is the primary site for metabolism of almost
all drugs (and other xenobiotics) because of its relative richness in
possessing a large variety of enzymes in large amounts. Metabolism by organs other
than liver (called as extrahepatic
metabolism) is of minor importance since
lower level of drug metabolising enzymes are present in such tissues. The
decreasing order of drug metabolising ability of various organs is:
Liver > Lungs > Kidneys
> Intestine > Placenta > Adrenals > Skin Brain, testes, muscles, spleen, etc. also metabolise drugs but to a
small extent.
Drug Metabolising Enzymes
The enzymes that biotransform xenobiotics differ
from those that metabolise food materials. They are versatile and non-specific
in metabolising a large number of drugs. The enzymes are broadly divided into 2
categories:
·
Microsomal enzymes
·
Non-microsomal enzymes.
The microsomal enzymes catalyse a majority of drug
biotransformation reactions. The microsomes are basically artefacts which
resulted when attempts were first made to isolate endoplasmic reticulum of the
liver homogenate. These vesicular fragments or microsomes are derived from
rough endoplasmic reticulum (rough due to the presence of RNA rich ribosomes on
the membrane surface whose function is protein synthesis) which shed their
ribosomes to become smooth surfaced. The large variety of microsomal enzymes
catalyse a number of oxidative, reductive and hydrolytic and glucuronidation
reactions.
Some important
characteristics of microsomal enzyme system are:
·
The intact nature of lipoidal
membrane bound enzyme of the microsomes is essential for its selectivity
towards lipid-soluble substrates.
·
A number of lipid-soluble
substrates (xenobiotics in general) can interact nonspecifically with the
microsomal enzymes. Natural endogenous substances which are generally
water-soluble do not interact.
·
The lipid soluble substrate is
biotransformed into a water-soluble metabolite by the microsomal enzymes which
can be readily excreted.
The non-microsomal
enzymes include those that are present in soluble form in the cytoplasm and
those attached to the mitochondria but not to endoplasmic reticulum. These are
also non-specific enzymes that catalyse few oxidative reactions, a number of
reductive and hydrolytic reactions and conjugation reactions other than
glucuronidation. It is interesting to note that, in contrast to microsomal
enzymes, the non-microsomal enzymes, especially the soluble enzymes, act on
relatively water-soluble xenobiotics (as well as endogenous compounds), e.g.
oxidases, peroxidases, dehydrogenases, esterases, etc.
Related Topics
