Gas Exchange
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Respiratory System
The alveoli in the lungs carry on the exchange of gases between air and the blood.
Gas
Exchange
The alveoli in the lungs carry on the exchange of gases
between air and the blood. They are microscopic air sacs clustered around the
distal ends of the narrow-est respiratory tubes called the alveolar ducts. Each alveolus consists of a tiny space inside a
thin wall, separating it from the adjacent alveoli. The inner lin-ing is made
up of simple squamous epithelium. Dense networks of capillaries are found near
each alveolus.
At least two thicknesses of epithelial cells and a fused
basement membrane layer separate the air in an alve-olus from the blood in a
capillary. These layers make up the respiratory
membrane. It is here where blood and alveolar air exchange gases.
The respiratory membrane consists of three layers:
■■ Squamous epithelial cells that line
each alveolus
■■ Endothelial cells that line
adjacent capillaries
■■ Fused basement membranes between
the alveolar and endothelial cells
Air exchanges across the respiratory membrane occur by the
diffusion process. Diffusion occurs from regions of higher pressure toward
regions of lower pressure. The pressure of a gas determines how it dif-fuses
from one region to another. Ordinary air con-sists of 78% nitrogen, 21% oxygen,
0.04% CO2, and traces of other gases. The amount of pressure each
gas contributes is called the partial
pressure of that gas. Because air is 21% oxygen, oxygen accounts for
21% of the atmospheric pressure, equivalent to 160 mm Hg of the atmospheric
pressure of 760 mm Hg.
The resulting concentration of each gas is pro-portional to
its partial pressure. Each gas diffuses between areas of higher partial
pressure and areas of lower partial pressure, until the two areas reach
equi-librium. CO2 diffuses from blood because of higher partial
pressure across the respiratory membrane and into alveolar air. Oxygen diffuses
from alveolar air into blood. Because of the large volume of air always present
in the lungs, as long as breathing continues, alveolar partial oxygen pressure
stays relatively con-stant at 104 mm Hg. The partial pressure of oxygen is
symbolized as Po2 and the partial pressure of CO2 is
symbolized as Pco2.
Dalton’s Law of Partial Pressures
Dalton’s
law of partial pressures states that the total pressure from a mixture of gases is the sum of
the pres-sure that each gas exerts independently. At any given moment, 78.6% of
the collisions between air molecules involve nitrogen molecules. The other
collisions that occur follow the presence of the specific molecules in the air:
20.9% are oxygen molecules, 0.5% are H2O molecules, and 0.04% are CO2
molecules. The com-bined effects of these collisions comprise atmospheric
pressure, which is 760 mm Hg.
Henry’s Law
Henry’s
law states that when a gas contacts a liquid, it dissolves in the liquid in proportion to its
partial pres-sure. During the gas phase, greater concentrations of a gas
cause it to go into the solution in the liquid in higher quantities and at a
faster rate.
The solubility of
a gas in a liquid along with the liquid’s temperature determines how much of
the gas will dissolve in the liquid at a specific partial pressure. From the
air, CO2 and other gases have various solubilities in H2O
or in blood plasma. CO2 has the highest solubility, with oxygen only
1/20th as soluble. Nitrogen is only half as soluble as this. Therefore, at a
certain partial pressure, nearly no nitrogen will dissolve in H2O,
but there is twice as much oxygen that will dissolve and 20 times more CO2
than this amount.
Gas solubility decreases as the temperature of a liquid
increases. For example, soda loses the CO2 that it contains more
rapidly at room temperature than it does in a refrigerator. Once all the CO2
escapes from the solution, all that is left is flavored H2O, lacking
any carbonation.
Diffusion and Respiratory Function
Diffusion of oxygen, CO2, and nitrogen between
gas and liquid forms follow the laws of how gases exist. Different partial
pressures and solubilities influence the direction and diffusion rate across
the respiratory membrane separating air inside the alveoli from blood inside
the alveolar capillaries.
The Composition of Alveolar Air
When air enters the respiratory tract, it begins to change
immediately. Inside the nasal cavity, inhaled air is warmed. H2O
vapor increases, with humidifi-cation and filtration continuing as air moves
through the pharynx, trachea, and bronchial passages. When the air reaches the
alveoli, it mixes with air that has remained inside the alveoli from the
previous respira-tory cycle. This means that alveolar air contains more CO2
and less oxygen than atmospheric air.
The final 150 mL of inhaled air does not get fur-ther than
the conducting passages, remaining in the anatomic dead space inside the lungs.
When the next exhalation occurs, the outward moving alveolar air mixes with
dead space air. This produces another mix-ture that is different from
atmospheric air as well as alveolar air.
Alveolar Gas Movement
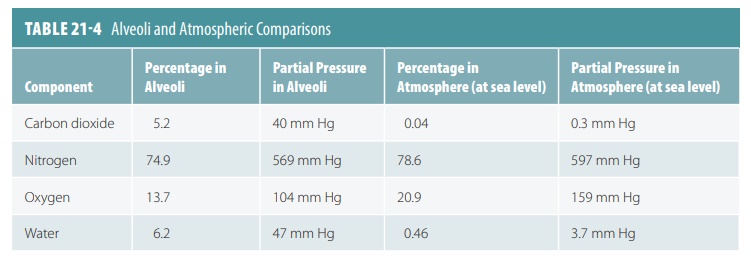
The mixture of gases in the atmosphere is very dif-ferent
from the mixture of gases in the alveoli of the lungs. TABLE 21-4 compares these differences. The
alve-oli contain more CO2 and H2O vapor and much less
oxygen than the atmosphere, which also has abundant amounts of nitrogen.
Three primary factors influence the amounts of gases in the alveoli and atmosphere. In the lungs, gas exchanges include the diffusion of oxygen from the alveoli into the pulmonary blood and the diffusion of CO2 in the opposite direction. The conducting pas-sages humidify the air inside them. With each breath, alveolar gases mix. Only 500 mL of air enter with every tidal inspiration. Therefore, alveolar gas is really a mixture of new, inspired gases with those that remain in the respiratory passages between breaths. Increas-ing the depth and rate of breathing easily changes the alveolar partial pressures of oxygen and CO2. A high AVR brings in more oxygen and the alveolar partial pressure of oxygen therefore increases. Also, CO2 is rapidly eliminated from the lungs.
1. Describe a spirometer.
2. Define the AVR.
3. Explain Dalton’s law.
4. Describe Henry’s law.
5. Identify
the composition of alveolar air.
External Respiration
External
respiration is also referred to as
pulmonary gas exchange. The blood in the pulmonary circuit is dark red in color. When it is returned
to the heart and oxygenated for distribution to the body tissues, it becomes
scarlet, which is much brighter red. This is because of the uptake of oxygen to
hemoglobin (Hb) in the
red blood cells. The unloading or exchange
of CO2 also occurs at the same time with the same speed. External
respiration is influenced by three factors: the respiratory membrane’s surface
area and thick-ness, gas solubilities and partial pressure gradients, and the
fact that alveolar ventilation is matched with pulmonary blood perfusion by
ventilation-perfusion coupling. In a normal lung, gas exchange is extremely
efficient, and the respiratory membrane is thin, only between 0.5 and 1 μm.
Internal Respiration
In internal
respiration, gas is exchanged between the capillaries and body tissues.
Diffusion gradients and partial pressure are reversed from those in exter-nal
respiration and pulmonary gas exchange. The ways in which gas exchanges occur
between the systemic capillaries and the body tissues are nearly the same,
however, as those occurring in the lungs. In the body tissue cells, CO2
is produced while oxygen is continu-ously used for metabolic processes.
The partial pressure of oxygen is always lower in the
tissues at 40 mm Hg than in the systemic blood, in which it is 100 mm Hg. This
means that oxygen quickly moves from the blood into the tissues, up to the
point that equilibrium is reached. Simultaneously, CO2 is quickly
moved along its pressure gradient into the blood. Therefore, in the venous
blood returning to the heart from the capillary beds, the partial pressure of
oxygen is 40 mm Hg, whereas the partial pressure of CO2 is 45 mm Hg.
Gas exchanges occurring between the blood and alveoli and between the blood and
body tissue cells occur via simple diffusion. This is influ-enced by the
partial pressure gradients of oxygen and CO2 on either side of the
exchange membranes.
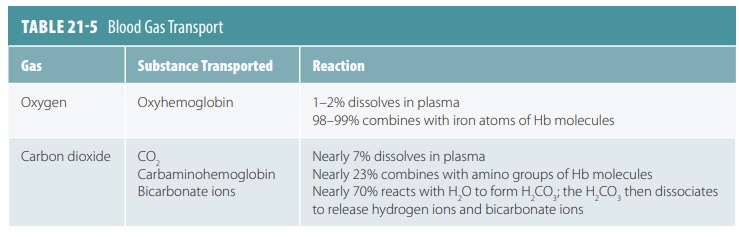
Oxygen Transport
As oxygen from the lungs and CO2 from the cells
enter the blood, they dissolve in the plasma or combine with blood components.
About 98% of the oxygen trans-ported by the blood binds the iron-containing
protein Hb in red blood cells. The remainder dissolves in the plasma. Because
oxygen is poorly soluble in H2O, only approximately 1.5% of
transported oxygen is carried in its dissolved form. This is why nearly all
oxygen transported from the lungs to the body tissues occurs via its chemical
combination with Hb.
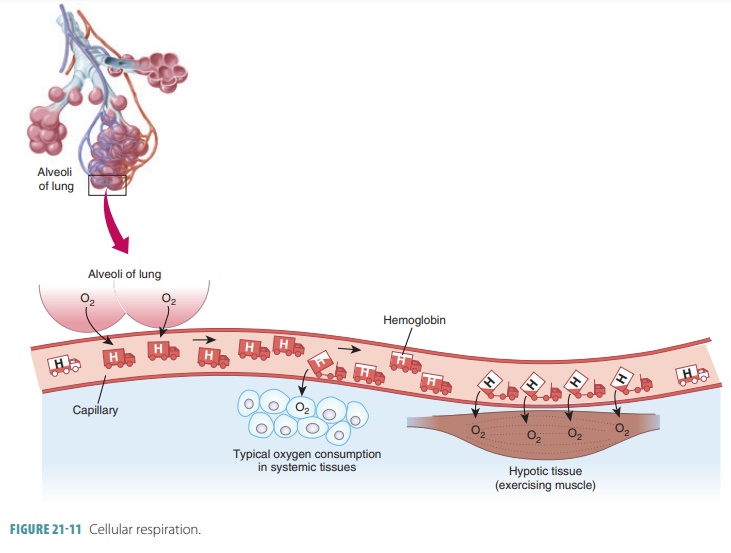
In the lungs, oxygen dissolves in blood and combines rapidly with the iron atoms of Hb to form oxyhemoglobin (HbO2), whose bonds are unstable. As PO2 decreases, HbO2 molecules release oxygen, diffusing into nearby cells that have depleted their oxygen supplies in cellular respiration ( FIGURE 21-11). Hb that has released oxygen is referred to as reduced Hb or deoxyhemoglobin (HHb). The loading and unload-ing of oxygen is described in the following reversible equation:
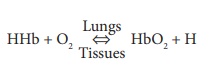
The Hb molecule changes shape after the first oxygen
molecule binds to iron. Then, it takes up two more oxygen molecules more
easily, with uptake of the fourth molecule still easier. An Hb molecule is partially saturated when one to three
oxygen mole-cules are bound. It is fully
saturated when all four of its heme groups are bound to oxygen. Unloading
of a single oxygen molecule enhances unloading of the next molecule and then
the next, meaning unloading and loading are functionally similar although
oppo-site processes. The binding strength or affinity of Hb for oxygen is altered based on how much oxygen
saturation exists. Both loading and unloading pro-cesses are extremely
efficient. The Hb
saturation and the partial pressure of oxygen saturation may be compared
using a graph called an oxygen-Hb
satura-tion curve (FIGURE
21-12).
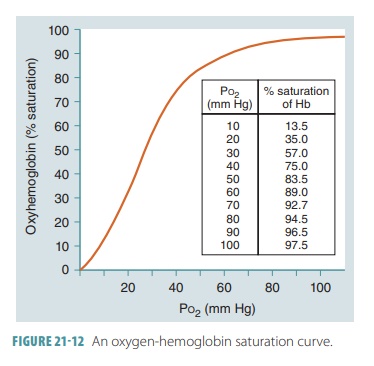
The partial pressure of oxygen, blood pH, tem-perature, the
partial pressure of CO2, and blood con-centrations of 2,3-bisphosphoglycerate all regulate the
rate of Hb reversibly binding or releasing oxygen. These interacting
determinants help deliver adequate amounts of oxygen to the body tissue cells.
As blood becomes more acidic or blood tempera-ture rises, CO2 increases in the blood, causing more release of oxygen. Therefore, during physical exercise, more oxygen is released to skeletal muscles. This increases CO2 contraction, decreases pH, and raises temperature.
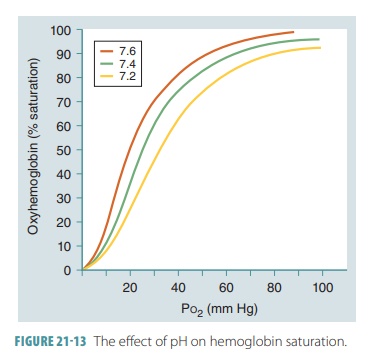
Hemoglobin and pH
At a certain partial pressure of oxygen or Po2,
Hb releases more oxygen if pH decreases. As shown in FIGURE 21-13, the oxygen -Hb saturation curve of
normal blood includes a pH of 7.4 and a temperature of 98.6°F (37°C) . Active
tissues not only consume oxygen, but also generate acids that lower
intersti-tial fluid pH. When this pH drops, Hb molecules change shape.
Therefore, they release their oxygen more easily and the slope of the Hb
saturation curve changes. Another way to understand this is to realize that
when pH drops, saturation declines. When the tissue partial pressure of oxygen
is 40 mm Hg, a pH drop from 7.4 to 7.2 reduces Hb sat-uration, from 75% to 60%.
The Hb molecules release 20% more oxygen in the peripheral tissues when the pH is
at 7.2 than when it is at 7.4. This relationship between pH and the Hb
saturation curve is called the Bohr
effect.
The primary compound influencing the Bohr effect is CO2. When it diffuses into blood, it quickly diffuses into red blood cells. The enzyme known as carbonic anhydrase catalyzes the reaction of CO2 with H2O molecules:
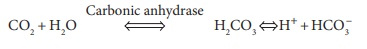
Because of this reaction, carbonic acid or H2CO3 is produced, since it dissociates into one hydrogen ion (h) and one bicarbonate ion (HCO3‒). The speed of H2CO3 formation is based on how much CO2 is pres-ent, which depends on the partial pressure of CO2 or Pco2. When this rises, H2CO3 formation increases, and the reaction occurs from left to right. Hydrogen ions that are created diffuse out of RBCs. The pH of the plasma then drops. When the Pco2 reduces, hydro-gen ions diffuse out of the plasma, and into the RCs. Therefore, the plasma pH rises, as the reaction occurs from right to left.
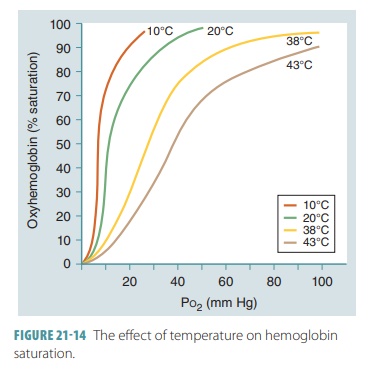
Hemoglobin and Temperature
At a certain partial pressure of oxygen, Hb releases more
oxygen if the temperature increases. The slope of the Hb saturation curve is
affected by temperature changes (FIGURE
21-14). More oxygen is released from Hb as temperatures rise. When
temperatures decline, Hb retains oxygen more tightly. The effects of
temperatures are only significant in active tissues that are generating a
great amount of heat. As active skeletal muscles generate heat, this heat warms
the blood flowing through them. As the blood increases in temperature, the Hb
molecules release more oxygen, which is utilized by the active muscle fibers.
Fetal Hemoglobin
In a developing fetus, the red blood cells contain fetal Hb. Its structure is
different from adult Hb and has
a much larger affinity for oxygen. At the same partial pressure of oxygen,
fetal Hb finds more oxygen than adult Hb (FIGURE 21-15). This is an important factor concerning the
transfer of oxygen across the placenta. The fetus obtains oxygen from the
maternal bloodstream, and at the placental, the maternal blood has a relatively
low Po2 ranging from 35 to 50 mm Hg.
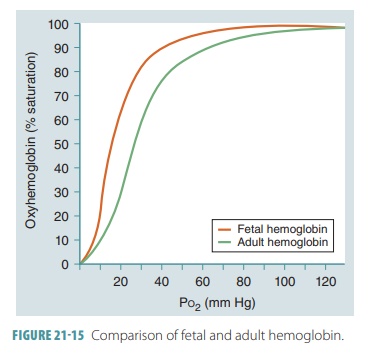
When maternal blood arrives at the placenta with its Po2
at 40 mm Hg, there is an Hb saturation of approximately 75%. Fetal blood
arriving at the pla-centa has close to 20 mm Hg for its Po2. Since
fetal Hb has a higher oxygen affinity, however, it is still at 58% saturation.
With diffusion occurring between fetal and maternal blood, oxygen enters the
blood-stream of the fetus until the Po2 reaches equilibrium (30 mm
Hg).
At this point, the maternal Hb is less than 60% saturated, while the fetal Hb is more than 80% saturated. The saturation curve’s steep slope regarding fetal Hb shows that when fetal RBCs reach the periph-eral tissues, a large amount of oxygen is released by the Hb molecules in response to only a very tiny change in Po2. At birth, a newborn takes its first breaths and the pressure in the lungs decreases.
Carbon Dioxide Transport
Blood transports CO2 to the lungs either as CO2
dis-solved in plasma, in quantities of 7–10%; as part of a compound formed by
bonding to Hb, which is slightly more than 20%; or converts H2CO3
to a bicarbon-ate ion, which is approximately 70%. FIGURE 21-16 explains CO2 transport. The amount of dissolved
CO2 in the plasma is determined by its partial pressure. The higher
the partial pressure of CO2 in the tissues, the more of it that will
go into solution. Only about 7% of CO2 transported by the blood is
in this form. Approx-imately 200 mL of CO2 are produced by active
body cells every minute. This is exactly the same amount that is excreted by
the lungs.
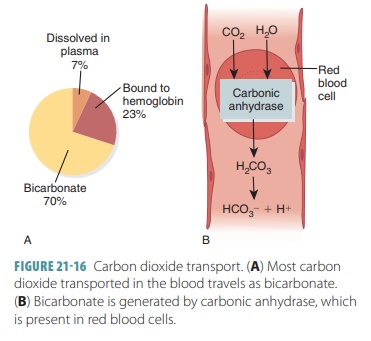
CO2 differs from oxygen in that it bonds with the
amino groups of the “globin” or protein portion of these molecules. Oxygen and
CO2 do not compete for binding sites. Hb can transport both
molecules at the same time. CO2 loosely bonds with Hb to slowly form
carbaminohemoglobin,
which decomposes readily in
regions of low CO2 partial pressure. This can be better understood
by the following equation:
CO2 + Hb ⇔ HbCO2
(carbaminohemoglobin)
No catalyst is needed for this rapid reaction. Because CO2
binds directly to the amino acids of globin and not to the heme, transport of
CO2 to red blood cells does not compete with HbO2
transport. The partial pressure of CO2 and degree of Hb oxygenation
directly influence loading and unloading of CO2. It quickly
dissociates from the Hb in the lungs, where the partial pressure of CO2
of air in the alveoli is lower than that in the blood. Deoxygenated Hb can
combine more quickly with CO2 than oxygenated Hb can combine with CO2.
The most important CO2 transport mechanism forms bicarbonate ions. CO2 reacts with H2O to form H2CO3.
Most CO2 molecules that enter the plasma quickly
enter the red blood cells. It is unstable, dissociating into hydro-gen and
bicarbonate ions, as seen in this equation:
CO2 + H2O ⇔ H2CO3
⇔ H+
+ HCO–3
In red blood cells, the enzyme carbonic anhydrase
speeds the reaction of CO2 and H2O, resulting in H2CO3 that
releases hydrogen and bicar-bonate ions. Nearly 70% of CO 2
transported by the blood is in this form. Because of carbonic anhydrase, the
reaction shown in the equation above occurs thou-sands of times faster in red
blood cells than it does in the plasma. The released hydrogen ions and the
released CO2 bind to Hb and trigger the Bohr effect. Therefore, CO2 loading enhances oxygen
release.
Because Hb acts as a buffer, freed hydrogen ions do not
cause a significant change in pH under resting conditions. Therefore, blood
only becomes slightly more acidic as it passes through tissues. Its pH declines
only from 7.4 to 7.34 in this process.
Generated bicarbonate ions move fast from red blood cells to
the plasma to be carried to the lungs. To balance this, chloride ions move from
the plasma into red blood cells. This process of ion exchange is known as the chloride shift. It occurs because of facilitated
diffusion, through a red blood cell membrane protein.
This process is reversed in the lungs. Bloodmoving through
the pulmonary capillaries experiences a decline in its partial pressure of CO2
from 45 to 40 mm Hg. CO2 is first released from bicarbonate
housings for this to occur. Bicarbonate ions reenter the RBCs, with chloride
ions moving into the plasma. The bicarbonate ions bind with hydrogen ions,
forming H2CO3, which is then split by carbonic anhydrase
to release H2O and CO2. This CO2 as well as
the remainder released from Hb and solution in plasma diffuses along its
partial pressure gradient from the blood into the alveoli.
CO2 diffuses into the alveoli in response to
rel-atively low partial pressure of CO2 in alveolar air. Hydrogen
and bicarbonate ions in red blood cells simultaneously recombine to form H2CO3,
quickly yielding CO2 and H2O. TABLE 21-5 summarizes how blood gases are transported.
1. What is the partial pressure of oxygen?
2. List potential causes of hypoxia.
3. Describe the effect of CO2 and hydrogen upon oxygen
unloading and name this effect.
4. Identify the three ways CO2 is transported in the blood and
name the most important of these ways.
5. Explain the relationship between CO2 and pH in the blood.