The Pleural Cavities and Pleural Membranes
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Respiratory System
The pleurae are relatively thin, even though they have two layers. Although they can slide across each other with ease, their separation is highly resisted by the surface tension of the pleural fluid.
The
Pleural Cavities and Pleural Membranes
The pleurae are
relatively thin, even though they have two layers. Although they can slide
across each other with ease, their separation is highly resisted by the surface
tension of the pleural fluid. Each pleura is a potential space and not an open
structure.
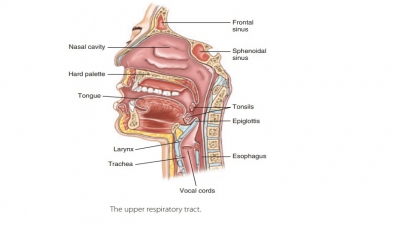
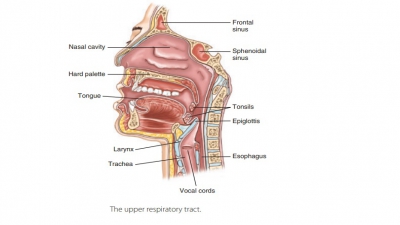
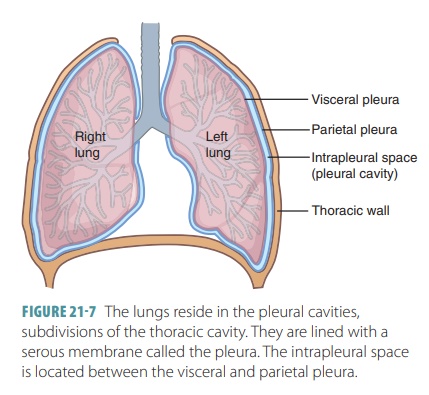
The visceral
pleura consists of a layer of serous membrane attached to each
lung surface that folds back to become the parietal pleura. The visceral pleura covers the
external lung surfaces and lines their fissures. The parietal pleura forms part
of the mediasti-num and lines the inner thoracic cavity (FIGURE 21-7). It also forms the superior face
of the diaphragm. The slit-like potential space between the visceral and
parietal pleurae is called the pleuralcavity. It contains a thin film of serous or pleural fluid that reduces friction as the
pleurae move against each other during breathing.
Pulmonary Ventilation
Pulmonary
ventilation is a mechanical process thatconsists of inspiration and expiration. Alveolar venti-lation
is the movement of air into and out of the alveoli. Pulmonary ventilation is
based on volume changes in the thoracic cavity. You should remember the
following:
■■ Volume changes always lead to
pressure changes.
■■ Pressure changes lead to flow of
gases to equalize pressure.
The relationship between pressure and volume of a gas is
explained by Boyle’s law, which
states that at a constant temperature, the pressure of a gas changes inversely
with its volume:
P1V1= P2V2
where P is the
pressure of the gas, V is its volume,
and 1 and 2 indicate the initial condition and the resulting condition,
respectively.
Gases always fill their containers, so the size of a
container influences the space between gas mole-cules. A larger container will
cause gas molecules to be farther apart and, therefore, pressure to be lower.
Reducing container volume brings the gas molecules closer and increases the
pressure. This is a simple way to understand how gases work inside our lungs.
An example that helps explain this principle is a car tire. The air inside the
tire is compressed to about one-third of its atmospheric volume, providing high
pres-sure that can handle the weight of the car.
Expiration
Normal individuals utilize quiet expiration in a passive
process, which is based more on the elasticity of the lungs than upon muscle
contraction. The rib cage descends and the lungs recoil as the inspiratory
muscles relax, resuming their resting lengths. Therefore, thoracic and
intrapulmonary volumes are decreased. This decrease in volume compresses the
alveoli. Intrapulmonary pressure rises to approximately 1 mm Hg higher than
atmospheric pressure. When the intrapulmonary pres-sure is greater than the
atmospheric pressure, the pres-sure gradient forces gases out of the lungs.
Forced
expiration is active and caused whenabdominal wall muscles contract.
These are mostly the oblique and transversus muscles. There are two results of
these contractions:
■■ Increased
intra-abdominal pressure: This forcesabdominal organs superiorly against
the diaphragm.
■■ Depression of the rib cage:
The internal intercostalmuscles assist in this depression and decrease thoracic
volume.
The accessory muscles of expiration require con-trol in
order to accurately regulate air flow from the lungs. The ability of a singer
to hold a long note is based on coordination of several muscles that are
usu-ally used for forced expiration.
1. Describe the force that controls pulmonary ventilation.
2. Identify the actions of the diaphragm and intercostal
muscles in inspiration.