Bone Homeostasis
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Support and Movement: Bone Tissues and the Skeletal System
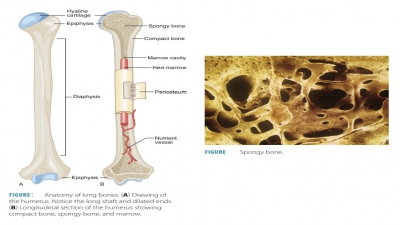
Bone homeostasis is the process of self-repair of the bones, which are active and dynamic types of tissue that undergo continual changes. Between 5% and 7% of bone mass is recycled every week.
Bone
Homeostasis
Bone homeostasis is the process
of self-repair of the bones, which are active and dynamic types of tissue that
undergo continual changes. Between 5% and 7% of bone mass is recycled every
week. Each day, up to 500 mg of calcium may enter or leave the skeleton of an
adult. Compact bone is replaced every 10 years or so, whereas spongy bone is
replaced every 3–4 years. These activities help to avoid bones becoming
brit-tle, which occurs when calcium salts slowly crystal-lize over time.
Brittle bones are much more likely to become fractured. Bone fracture is the
most common disorder of bone homeostasis.
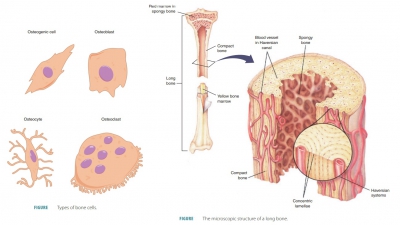
Bone Remodeling
The surfaces of a bone’s
periosteum and endosteum experience both bone deposit and resorption in adults.
Bone deposit and resorption make up the process of bone
remodeling. Groups of nearby
osteoblasts and osteoclasts form remodeling
units. These units control bone remodeling, assisted by osteocytes, which
sense bone stressors. Total bone mass is constant in healthy, younger adults.
This means that rates of bone deposit and resorption are in balance. Remodeling
occurs in different amounts in various bones. Although the shaft of the femur
(thighbone) is remodeled slowly, its distal area is completely replaced within
six months.
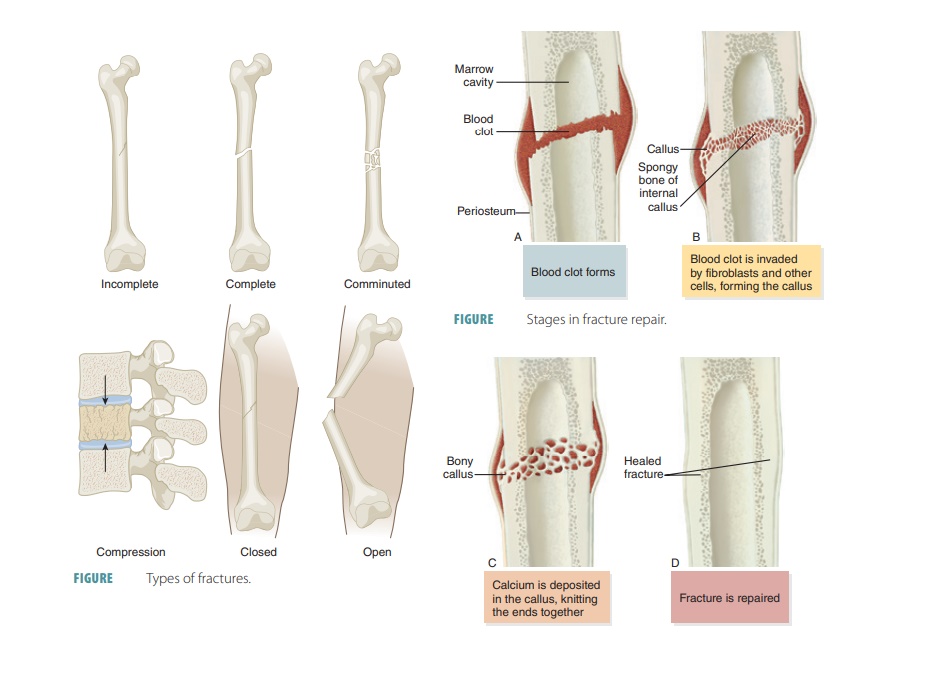
Bone Fracture
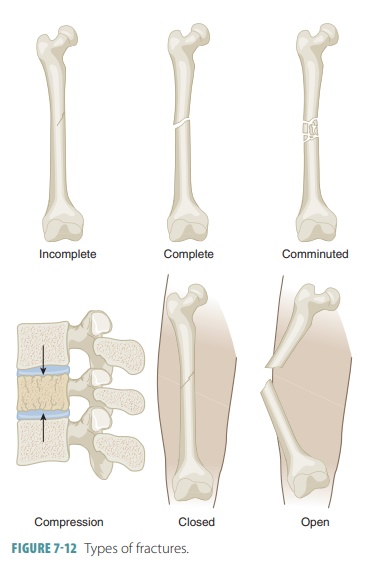
Bone fractures are classified as
positioning, complete-ness of the fracture, and penetration of the skin by the
bones. Positioning of fractures includes classifications such as nondisplaced fractures and displaced fractures. A nondisplaced
fracture means the bone ends remain in their normal position and a displaced
fracture means the bones are out of their normal alignment. Regarding the
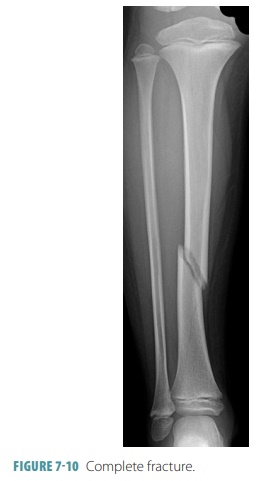
completeness of the fracture, a complete
fracture describes one in which the bone is broken completely through (FIGURE 7- 10),
whereas an incomplete fracture means
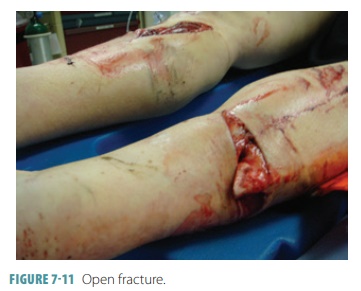
the bone is not broken completely through. If a bone penetrates the skin, it is
called an open or com-pound fracture (FIGURE 7-11). If
not, it is called a closed or simple fracture. Fractures can also be
described in terms of their location, their external appearance, and the manner
in which the bone is broken:
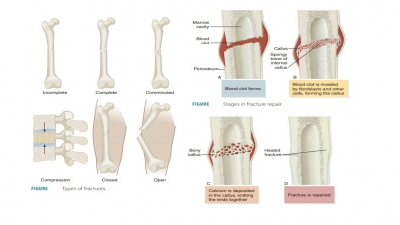
■■ Comminuted
fracture: This type is where the bone has fragmented into three or more pieces.
This type is more common in elderly people, whose bones may be brittle.
■■ Compression fracture:The
bone has been crushed. This type is common in porous bones such as when the
condition known as osteopo-rosis exists or when a person falls from a great
height, causing extreme bone trauma, an exam-ple is when vertebrae are
subjected to extreme vertical stress.
■■ Depressed
fracture: The broken bone portion is pressed inward, as often occurs in skull
fractures.
■■ Epiphyseal
fracture: The epiphysis has separated from the diaphysis along the epiphyseal
plate. This type of fracture often occurs where cartilage cells are dying and
the matrix is becoming calcified.
■■ Greenstick
fracture: The bone breaks incompletely, with breakage occurring only on one
side of the shaft, whereas the other side bends. This type of fracture is
common in children, because their bones have more organic matrix, lending more
flexibility than the bones of adults.
■■ Spiral fracture: Because
of excessive twisting forces, a ragged bone break occurs. This type of fracture
commonly occurs due to sports activities. Treatment of bone fractures requires
reduction, which is the realignment of the broken bone ends. A closed or
external reduction requires the physician to physically manipulate the bone
ends into position. An open or internal reduction requires the bone ends to be
pulled together surgically, using pins or wires. After reduction, the broken
bones are immobilized by a cast or traction. In a young adult, a simple
fracture of a small or medium-sized bone will heal within eight weeks.
How-ever, the break of a larger, weight-bearing bone requires a much longer
time to heal. In an elderly person, because of their reduced circulation, bone
fractures always take longer to heal compared with younger adults. Various
classifications of fractures are shown in FIGURE 7-12.
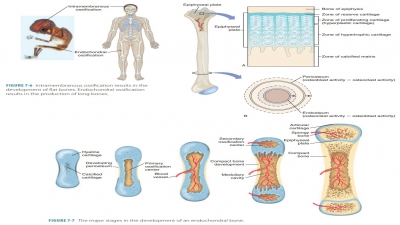
Bone Repair
For simple bone fractures, bone
repair involves four primary stages (FIGURE
7-13): hematoma formation, formation
of a fibrocartilaginous callus, formation of a bony callus, and the process of
bone remodeling:
■■ Hematoma formation: The
fracture of a bone causes bone and periosteum blood vessels to hemorrhage. A
hematoma forms at the fracture site. This is also referred to as a fracture
hematoma. Because of a lack of nutrients, bone cells die, and the area of the
fracture becomes inflamed, painful, and swollen.
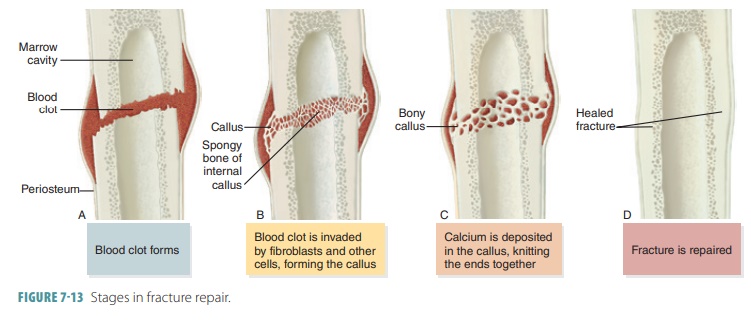
■■ Fibrocartilaginous callus
formation: A soft fibrocartilaginous callus (soft granulation tissue) forms in
a few days, with capillaries growing into the hematoma. This is also known as
aninternal callus. Phagocytes engulf debris as fibroblasts, cartilage, and
osteogenic cells begin bone reconstruction. Collagen fibers are produced by the
fibroblasts, spanning the break and connecting the bone ends. Certain precursor
cells differentiate into chondroblasts, secreting cartilage matrix. Inside the
tissue repair mass, osteoblasts start to form spongy bone. Cartilage cells at
the furthest point from the capillaries secrete a cartilaginous matrix, which
bulges and eventually calcify. This entire repair tissue mass is called the
fibrocartilaginous callus and splints broken bones.
■■ Bony callus formation: New
bone trabeculae appear in the
fibrocartilaginous callus within one week. They slowly convert it to a hard, bony callus
of spongy bone. This is also known as an external callus. This process continues
until a union has become firm and requires about two
months. Basically, this process duplicates the events of endochondral
ossification.
■■ Bone remodeling: The bony callus is remodeled during bony callus formation. This continues for several months. Excess material inside the med-ullary cavity and on the diaphysis is removed. Compact bone is laid down, rebuilding bone shaft walls. Eventually, the final repaired bone structure appears nearly identical to the original unbroken region, because it responds to the same forms of mechanical stress.
Bone Deposition
Areas of new bone matrix deposits
are signified by oste-oid seams,
unmineralized sections of thin bone matrix,
only 10–12 μm in width. Between osteoid seams and older bone, an abrupt
transition point exists, known as the calcification
front. The osteoid seams mature for about one week before calcification
occurs. Mechanical signals are involved in this calcification. In the
endos-teal cavity, nearby concentrations of calcium and phos-phate ions are
needed for calcification to occur. When this calcium–phosphate product is
sufficient, tiny crystals of hydroxyapatites are formed. They catalyze
continued crystallization of calcium salts. Additional factors include matrix
proteins (which bind and con-centrate calcium) and alkaline
phosphatase, an enzyme that is lost in matrix vesicles by osteoblasts, and
which is critical for mineralization. Eventually, calcium salts are deposited
at one time throughout the matured matrix in an ordered manner.
Bone Resorption
Bone
resorption occurs because of
osteoclast activ-ities, including the creation of grooves or depressions as
bone matrix is broken down. Osteoclast borders use their irregular shape to
stick to the bone and seal off areas of bone destruction. They secrete
lysosomal enzymes, digesting protons and the organic matrix. In the resorption
bays, the high acidity converts calcium salts into soluble forms. Dead
osteocytes and deminer-alized matrix may be phagocytized by the osteoclasts.
Endocytosis occurs to the digested growth factors, matrix end products, and
dissolved minerals. These are moved, via transcytosis, across the osteoclasts
to be released at the opposite end, entering the intersti-tial fluid and blood.
Osteoclasts undergo apoptosis after a certain bone area has been resorbed.
Both parathyroid hormone
(PTH) and protein from the immune system’s T cells play a role.
Control of Bone Remodeling
Bone remodeling is controlled by
genetic factors, a negative feedback hormonal loop, and in response to
gravitational and mechanical forces. The negative feed-back hormonal loop
maintains calcium ion homeo-stasis in the blood. Ionic calcium is essential
for nerve impulse transmission, blood coagulation, muscle con-traction, cell
division, and secretion by glands as well as nerve cells. More than 99% of the
body’s 1,200–1,400 g of calcium is present in the bones. The remainder is
primarily in the cells and a small amount is in the blood. Hormonal controls
keep blood calcium ions in a range between 9 and 11 mg/dL (100 mL) of blood.
Vitamin D metabolites control calcium absorp-tion from the intestine.
Children under age 10 need 400–800 mg of calcium in their daily diet, whereas
people between ages 11 and 24 require 1,200–1,500 mg.
Parathyroid Hormone
PTH is released by the
parathyroid glands. It is the primary hormonal controller of bone remodeling,
although calcitonin is also involved to a lesser degree, elevated levels of calcium ions in
the blood stimulate the secretion of the hormone calcitonin. The parathy-roid
glands are embedded in the thyroid gland in the neck. PTH is released when
blood levels of ionic cal-cium decline, stimulating osteoclasts to resorb bone
and releasing calcium into the blood. Osteoclasts break down old as well as new
bone matrix. Rising blood calcium causes PTH release to stop, reversing its
effects and lowering blood calcium. Blood calcium homeostasis is, therefore,
maintained. However, if blood calcium levels are low for a long time, the bones
lose minerals and develop large, irregular holes.
Both bone density and bone
turnover react to a variety of hormones. The adipose tissue releases the
hormone leptin, which helps to
regulate bone density, weight, and energy balance. Leptin appears to inhibit
the actions of osteoblasts, via mediation by the hypothal-amus, activating
sympathetic nerves that serve bones. The balance between bone destruction and
formation are regulated by interactive processes of the brain, skel-eton, and
intestine. Serotonin mediates these
processes and is primarily manufactured in the intestine. It can-not enter the
brain because of the blood–brain barrier. Serotonin is secreted during eating,
circulating to the bones to interfere with osteoblast activity. Because bone
turnover is reduced after eating, calcium may be held in bones while new
calcium is moving into the blood-stream. Serotonin uptake inhibitors make
excessive serotonin available to bone cells, resulting in lower bone density
and higher potential for fracture.
Bone remodeling is also
controlled by gravity and mechanical stressors. According to Wolff’s law, bones grow or remodel in
response to demands placed on them. The anatomy of a bone is related to its
com-mon stressors. A bone is stressed when muscles pull on it or weight bears
down on it. These stressors are usually not centered and cause bending of the
bone. On one side, the bone is compressed by bending, whereas on the other
side, it experiences tension (stretching). Long bones are, therefore, thickest
at the midpoint of the diaphysis because this is where bending stresses are
strongest. Toward the center of the bone, compression and tension are lowest
because of their opposition of each other’s effects. Without causing any
serious complications, a bone can be hollowed out for lightness, which means
using spongy bone rather than compact bone. Wolff’s
law has several other points:
■■ Curved bones are thickest at the point where they are most likely to
break.
■■ Whether you are
right or left handed (handedness) determines the bones of the most-used upper
limb to be thicker than those of the less-used upper limb. Large increases in bone
strength occur from vigorous exercise of the most-used upper limb.
■■ Where heavy and
active muscles attach, large and bony projections develop.
■■ Spongy bone
trabeculae form a supportive frame work along compression lines.
■■ When bones are not
stressed, such as in a fetus or an immobilized patient, the bones lack normal
features. Deformation of a bone causes an electrical current, with compressed
and stretched regions having oppo-site charges. Therefore, it is believed by
some experts that electrical signals are in control of bone remodel-ing. Within
the canaliculi, the flowing of fluids appears to provide stimuli that control
bone remodeling.
Both hormonal and
mechanical factors affect the skeleton continuously. Hormonal controls, in
response to changing levels of blood calcium, deter-mine when (and if )
remodeling occurs. The location where remodeling occurs is determined by
mechanical stressors. When bone is required to be broken down to increase blood
calcium, PTH is released, targeting osteoclasts. Mechanical forces determine
which of the osteoclasts will be most sensitive to PTH. Therefore, bone in
areas of lowest stress is broken down because it is temporarily not essential
to the body.
1. Describe
the borders created by osteoclast activity and their significance.
2. For
maintaining blood calcium levels needed for bone homeostasis, is PTH or
mechanical stressors more important as a stimulus?
3. Differentiate
between bone growth and bone remodeling.
Related Topics