Chapter Summary, Study Questions
| Home | | Biochemistry |Chapter: Biochemistry : Enzymes
Enzymes are protein catalysts that increase the velocity of a chemical reaction by lowering the energy of the transition state.
CHAPTER SUMMARY
Enzymes are protein
catalysts that increase the velocity of a chemical reaction by lowering the
energy of the transition state (Figure 5.23). Enzymes are not consumed during
the reaction they catalyze. Enzyme molecules contain a special pocket or cleft
called the active site. The active site contains amino acid side chains that
participate in substrate binding and catalysis. The active site binds the
substrate, forming an enzyme–substrate (ES) complex. Binding is thought to
cause a conformational change in the enzyme (induced fit) that allows
catalysis. ES is converted to enzyme-product (EP), which subsequently
dissociates to enzyme and product. An enzyme allows a reaction to proceed
rapidly under conditions prevailing in the cell by providing an alternate reaction
pathway with a lower free energy of activation. The enzyme does not change the
free energies of the reactants or products and, therefore, does not change the
equilibrium of the reaction. Most enzymes show Michaelis-Menten kinetics, and a
plot of the initial reaction velocity (vo) against substrate
concentration ([S]) has a hyperbolic shape similar to the oxygen-dissociation
curve of myoglobin. Any substance that can diminish the velocity of such
enzyme-catalyzed reactions is called an inhibitor. The two most commonly
encountered types of reversible inhibition are competitive (which increases the
apparent Km) and noncompetitive (which decreases the apparent Vmax).
In contrast, the multisubunit allosteric enzymes frequently show a sigmoidal
curve similar in shape to the oxygen-dissociation curve of hemoglobin. They
typically catalyze the rate-limiting (slowest step) of a pathway. Allosteric
enzymes are regulated by molecules called effectors that bind noncovalently at
a site other than the active site. Effectors can be either positive (accelerate
the enzyme-catalyzed reaction) or negative (slow down the reaction). An
allosteric effector can alter the affinity of the enzyme for its substrate,
modify the maximal catalytic activity of the enzyme, or both. Enzymes can also
be regulated by covalent modification and by changes in the rate of synthesis
or degradation. Enzymes have diagnostic and therapeutic value in medicine.

Figure 5.23 Key concept map
for the enzymes. S = substrate; [S] = substrate concentration; P = product; E =
enzyme; vo = initial velocity; Vmax = maximal velocity; Km
= Michaelis constant; K0.5 = substrate concentration which gives
half maximal velocity.
Study Questions
Choose the ONE best answer.
5.1 In cases of ethylene glycol poisoning and its
characteristic metabolic acidosis, treatment involves correction of the
acidosis, removal of any remaining ethylene glycol, and administration of an
inhibitor of alcohol dehydrogenase (ADH), the enzyme that oxidizes ethylene
glycol to the organic acids that cause the acidosis. Ethanol (grain alcohol)
frequently is the inhibitor given to treat ethylene glycol poisoning. Results
of experiments using ADH with and without ethanol are shown to the right. Based
on these data, what type of inhibition is caused by the ethanol?
A. Competitive
B. Feedback
C. Irreversible
D. Noncompetitive
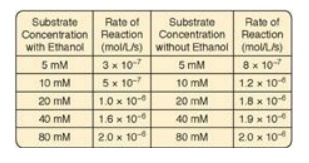
Correct answer = A competitive inhibitor increases the
apparent Km for a given substrate. This means that, in the presence of a
competitive inhibitor, more substrate is needed to achieve 1⁄2 Vmax.
The effect of a competitive inhibitor is reversed by increasing substrate
concentration ([S]). At a sufficiently high [S], the reaction velocity reaches
the Vmax observed in the absence of inhibitor.
5.2 ADH requires oxidized nicotinamide adenine
dinucleotide (NAD+) for catalytic activity. In the reaction
catalyzed by ADH, an alcohol is oxidized to an aldehyde as NAD+ is
reduced to NADH and dissociates from the enzyme. The NAD+ is
functioning as a (an):
A. apoenzyme.
B.
coenzyme-cosubstrate.
C. coenzyme-prosthetic
group.
D. cofactor.
E. heterotropic
effector.
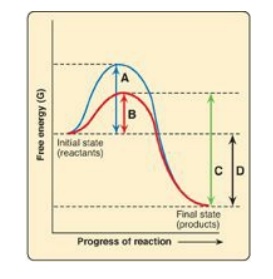
For Questions 5.3 and
5.4, use the graph below which shows the changes in free energy when a reactant
is converted to a product in the presence and absence of an enzyme. Select the
letter that best represents:
Correct answer = B. Coenzymes-cosubstrates are small
organic molecules that associate transiently with an enzyme and leave the
enzyme in a changed form. Coenzyme-prosthetic groups are small organic
molecules that associate permanently with an enzyme and are returned to their
original form on the enzyme. Cofactors are metal ions. Heterotropic effectors
are not substrates.
5.3 The free energy of activation of the catalyzed
forward reaction.
Correct answers = B; D. Enzymes (biocatalysts) provide an
alternate reaction pathway with a lower free energy of activation. However,
they do not change the free energy of the reactant or product. A is the free
energy of the uncatalyzed reaction. C is the free energy of the catalyzed
reverse reaction.
5.4 The free energy of the reaction.
Related Topics
