Collagen
| Home | | Biochemistry |Chapter: Biochemistry : Fibrous Proteins
Collagen is the most abundant protein in the human body. A typical collagen molecule is a long, rigid structure in which three polypeptides (referred to as α chains) are wound around one another in a rope-like triple helix.
COLLAGEN
Collagen is the most
abundant protein in the human body. A typical collagen molecule is a long,
rigid structure in which three polypeptides (referred to as α chains) are wound
around one another in a rope-like triple helix (Figure 4.1). Although these molecules
are found throughout the body, their types and organization are dictated by the
structural role collagen plays in a particular organ. In some tissues, collagen
may be dispersed as a gel that gives support to the structure, as in the
extracellular matrix or the vitreous humor of the eye. In other tissues,
collagen may be bundled in tight, parallel fibers that provide great strength,
as in tendons. In the cornea of the eye, collagen is stacked so as to transmit
light with a minimum of scattering. Collagen of bone occurs as fibers arranged
at an angle to each other so as to resist mechanical shear from any direction.
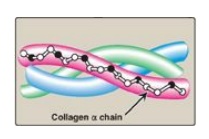
Figure 4.1 Triple-stranded
helix of collagen.
A. Types
The collagen
superfamily of proteins includes more than 25 collagen types as well as
additional proteins that have collagen-like domains. The three polypeptide α
chains are held together by interchain hydrogen bonds. Variations in the amino
acid sequence of the α chains result in structural components that are about
the same size (approximately 1,000 amino acids long) but with slightly
different properties. These α chains are combined to form the various types of
collagen found in the tissues. For example, the most common collagen, type I,
contains two chains called α1 and one chain called α2 (α12α2),
whereas type II collagen contains three α1 chains (α13). The
collagens can be organized into three groups, based on their location and
functions in the body (Figure 4.2).
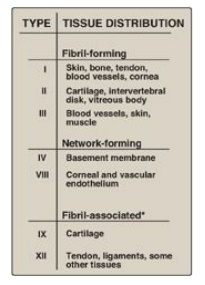
Figure 4.2
The most abundant types of collagen. *Known as FACITs: fibril-associated
collagens with interrupted triple helices.
1. Fibril-forming collagens: Types I, II, and III are the
fibrillar collagens and have the rope-like structure described above for a
typical collagen molecule. In the electron microscope, these linear polymers of
fibrils have characteristic banding patterns, reflecting the regular staggered
packing of the individual collagen molecules in the fibril (Figure 4.3). Type I
collagen fibers (composed of collagen fibrils) are found in supporting elements
of high tensile strength (for example, tendon and cornea), whereas fibers
formed from type II collagen molecules are restricted to cartilaginous
structures. The fibers derived from type III collagen are prevalent in more
distensible tissues such as blood vessels.
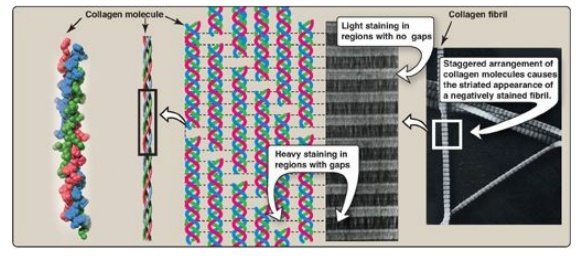
Figure 4.3 Collagen fibrils at right have a characteristic banding pattern, reflecting the regularly staggered packing of the individual collagen molecules in the fibril.
2. Network-forming collagens: Types IV and VIII form a
three-dimensional mesh, rather than distinct fibrils (Figure 4.4). For example,
type IV molecules assemble into a sheet or meshwork that constitutes a major
part of basement membranes.
Basement membranes are thin, sheet-like structures that provide mechanical support for adjacent cells and function as a semipermeable filtration barrier to macromolecules in organs such as the kidney and the lung.
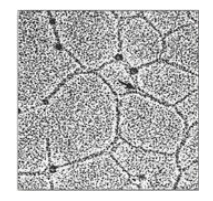
Figure 4.4 Electron micrograph of a polygonal network formed by association of collagen type IV monomers.
3. Fibril-associated collagens: Types IX and XII bind to the surface of collagen fibrils, linking these fibrils to one another and to other components in the extracellular matrix (see Figure 4.2).
B. Structure
1. Amino acid sequence: Collagen is rich in proline and glycine, both of which are important in the formation of the triple-stranded helix. Proline facilitates the formation of the helical conformation of each α chain because its ring structure causes “kinks” in the peptide chain. [Note: The presence of proline dictates that the helical conformation of the α chain cannot be an α helix.] Glycine, the smallest amino acid, is found in every third position of the polypeptide chain. It fits into the restricted spaces where the three chains of the helix come together. The glycine residues are part of a repeating sequence, –Gly–X–Y–, where X is frequently proline, and Y is often hydroxyproline (but can be hydroxylysine, Figure 4.5). Thus, most of the α chain can be regarded as a polytripeptide whose sequence can be represented as (–Gly–Pro–Hyp–)333.
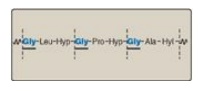
Figure 4.5 Amino acid sequence of a portion of the α1 chain of collagen. [Note: Hyp is hydroxyproline, and Hyl is hydroxylysine.]
2. Triple-helical structure: Unlike most globular proteins that are folded into compact structures, collagen, a fibrous protein, has an elongated, triple-helical structure that is stabilized by interchain hydrogen bonds.
3. Hydroxyproline and hydroxylysine: Collagen contains hydroxyproline and hydroxylysine, which are not present in most other proteins. These residues result from the hydroxylation of some of the proline and lysine residues after their incorporation into polypeptide chains (Figure 4.6). The hydroxylation is, thus, an example of posttranslational modification. [Note: Generation of hydroxyproline maximizes formation of interchain hydrogen bonds that stabilize the triple-helical structure.]
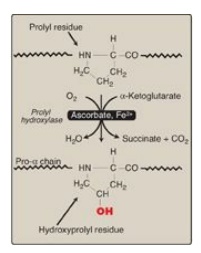
Figure 4.6 Hydroxylation of proline residues of pro-α chains of collagen by prolyl hydroxylase.
4. Glycosylation: The hydroxyl group of the hydroxylysine residues of collagen may be enzymatically glycosylated. Most commonly, glucose and galactose are sequentially attached to the polypeptide chain prior to triple-helix formation (Figure 4.7).
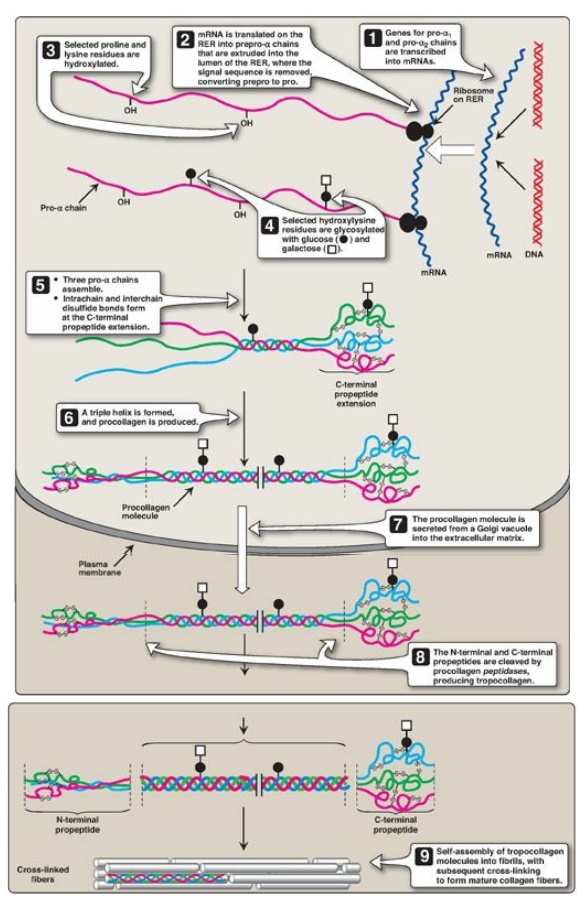
Figure 4.7 Synthesis of collagen. RER = rough endoplasmic reticulum; mRNA = messenger RNA. Synthesis of collagen.
C. Biosynthesis
The polypeptide
precursors of the collagen molecule are synthesized in fibroblasts (or in the
related osteoblasts of bone and chondroblasts of cartilage). They are
enzymically modified and form the triple helix, which gets secreted into the
extracellular matrix. After additional enzymic modification, the mature
extracellular collagen monomers aggregate and become cross-linked to form
collagen fibers.
1. Formation of pro-α chains: Collagen is one of many proteins
that normally function outside of cells. Like most proteins produced for
export, the newly synthesized polypeptide precursors of α chains (prepro-α
chains) contain a special amino acid sequence at their N-terminal ends. This
sequence acts as a signal that, in the absence of additional signals, targets
the polypeptide being synthesized for secretion from the cell. The signal
sequence facilitates the binding of ribosomes to the rough endoplasmic reticulum
(RER), and directs the passage of the prepro-α chain into the lumen of the RER.
The signal sequence is rapidly cleaved in the RER to yield a precursor of
collagen called a pro-α chain (see Figure 4.7).
2. Hydroxylation: The pro-α chains are processed by a
number of enzymic steps within the lumen of the RER while the polypeptides are
still being synthesized (see Figure 4.7). Proline and lysine residues found in
the Y-position of the –Gly–X–Y– sequence can be hydroxylated to form hydroxyproline
and hydroxylysine residues. These hydroxylation reactions require molecular
oxygen, Fe2+, and the reducing agent vitamin C, without which the hydroxylating
enzymes, prolyl hydroxylase and lysyl hydroxylase, are unable to function (see
Figure 4.6). In the case of ascorbic acid deficiency (and, therefore, a lack of
proline and lysine hydroxylation), interchain H-bond formation is impaired, as
is formation of a stable triple helix. Additionally, collagen fibrils cannot be
cross-linked (see below), greatly decreasing the tensile strength of the
assembled fiber. The resulting deficiency disease is known as scurvy. Patients
with ascorbic acid deficiency also often show bruises on the limbs as a result
of subcutaneous extravasation (leakage) of blood due to capillary fragility
(Figure 4.8).
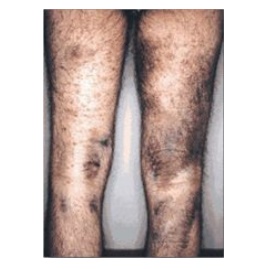
Figure 4.8 The legs of a 46-year-old man with scurvy.
3. Glycosylation: Some hydroxylysine residues are modified by
glycosylation with glucose or glucosyl-galactose (see Figure 4.7).
4. Assembly and secretion: After hydroxylation and
glycosylation, three pro-α chains form procollagen, a precursor of collagen
that has a central region of triple helix flanked by the nonhelical amino- and
carboxyl-terminal extensions called propeptides (see Figure 4.7). The formation
of procollagen begins with formation of interchain disulfide bonds between the
C-terminal extensions of the pro-α chains. This brings the three α chains into
an alignment favorable for helix formation. The procollagen molecules move
through the Golgi apparatus, where they are packaged in secretory vesicles. The
vesicles fuse with the cell membrane, causing the release of procollagen
molecules into the extracellular space.
5. Extracellular cleavage of procollagen molecules: After their release, the procollagen molecules are cleaved by N- and C-procollagen peptidases, which remove the terminal propeptides, releasing triple-helical tropocollagen molecules.
6. Formation of collagen fibrils: Tropocollagen molecules
spontaneously associate to form collagen fibrils. They form an ordered,
overlapping, parallel array, with adjacent collagen molecules arranged in a
staggered pattern, each overlapping its neighbor by a length approximately
three-quarters of a molecule (see Figure 4.7).
7. Cross-link formation: The fibrillar array of collagen
molecules serves as a substrate for lysyl oxidase. This Cu2+-containing extracellular
enzyme oxidatively deaminates some of the lysine and hydroxylysine residues in
collagen. The reactive aldehydes that result (allysine and hydroxyallysine) can
condense with lysine or hydroxylysine residues in neighboring collagen
molecules to form covalent cross-links and, thus, mature collagen fibers
(Figure 4.9).
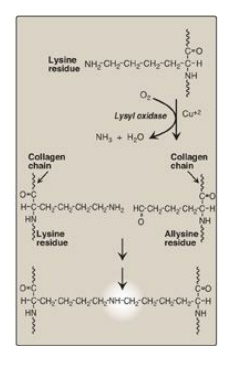
Figure 4.9 Formation of
cross-links in collagen. [Note: Lysyl oxidase is irreversibly inhibited by a
toxin from plants in the genus Lathyrus, leading to a condition known as
lathyrism.]
Lysyl oxidase is one of several copper-containing
enzymes. Others include cytochrome oxidase, dopamine hydroxylase, superoxide
dismutase, and tyrosinase. Disruption in copper homeostasis causes copper
deficiency (X-linked Menkes disease) or overload (Wilson disease).
D. Degradation
Normal collagens are
highly stable molecules, having half-lives as long as several years. However,
connective tissue is dynamic and is constantly being remodeled, often in
response to growth or injury of the tissue. Breakdown of collagen fibers is
dependent on the proteolytic action of collagenases, which are part of a large
family of matrix metalloproteinases. For type I collagen, the cleavage site is
specific, generating three-quarter and one-quarter length fragments. These
fragments are further degraded by other matrix proteinases.
E. Collagen diseases: Collagenopathies
Defects in any one of the many steps in collagen fiber synthesis can result in a genetic disease involving an inability of collagen to form fibers properly and, therefore, an inability to provide tissues with the needed tensile strength normally provided by collagen. More than 1,000 mutations have been identified in 23 genes coding for 13 of the collagen types. The following are examples of diseases that are the result of defective collagen synthesis.
1. Ehlers-Danlos syndrome: Ehlers-Danlos syndrome (EDS) is a heterogeneous group of connective tissue disorders that result from inheritable defects in the metabolism of fibrillar collagen molecules. EDS can be caused by a deficiency of collagen-processing enzymes (for example, lysyl hydroxylase or N-procollagen peptidase) or from mutations in the amino acid sequences of collagen types I, III, or V. The classic form of EDS, caused by defects in type V collagen, is characterized by skin extensibility and fragility and joint hypermobility (Figure 4.10). The vascular form, due to defects in type III collagen, is the most serious form of EDS because it is associated with potentially lethal arterial rupture. [Note: The classic and vascular forms show autosomal dominant inheritance.] Collagen containing mutant chains may have altered structure, secretion, or distribution. It frequently is degraded. [Note: Incorporation of just one mutant chain may result in degradation of the triple helix. This is known as a dominant-negative effect.].
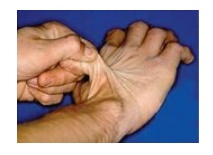
Figure 4.10 Stretchy skin of classic Ehlers-Danlos syndrome.
2. Osteogenesis imperfecta: This syndrome, known as brittle bone disease, is a genetic disorder of bone fragility characterized by bones that fracture easily, with minor or no trauma (Figure 4.11). Over 80% of cases of osteogenesis imperfecta (OI) are caused by dominant mutations to the genes that code for the α1 or α2 chains in type I collagen. The most common mutations cause the replacement of glycine (in –Gly–X–Y–) by amino acids with bulky side chains. The resultant structurally abnormal α chains prevent the formation of the required triple-helical conformation. Phenotypic severity ranges from mild to lethal. Type I OI, the most common form, is characterized by mild bone fragility, hearing loss, and blue sclerae. Type II, the most severe form, is typically lethal in the perinatal period as a result of pulmonary complications. In utero fractures are seen (see Figure 4.11). Type III is also a severe form. It is characterized by multiple fractures at birth, short stature, spinal curvature leading to a “humped-back” (kyphotic) appearance, and blue sclerae. Dentinogenesis imperfecta, a disorder of tooth development, may be seen in OI.
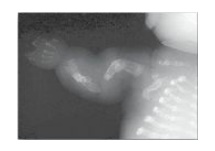
Figure 4.11 Lethal form (type II) of osteogenesis imperfecta in which the fractures appear in utero, as revealed by this radiograph of a stillborn fetus.
