Properties of Enzymes
| Home | | Biochemistry |Chapter: Biochemistry : Enzymes
Enzymes are protein catalysts that increase the velocity of a chemical reaction and are not consumed during the reaction.
Enzymes are protein
catalysts that increase the velocity of a chemical reaction and are not
consumed during the reaction. [Note: Some RNAs can act like enzymes, usually
catalyzing the cleavage and synthesis of phosphodiester bonds. RNAs with
catalytic activity are called ribozymes and are much less commonly encountered
than protein catalysts.]
A. Active sites
Enzyme molecules
contain a special pocket or cleft called the active site. The active site,
formed by folding of the protein, contains amino acid side chains that
participate in substrate binding and catalysis (Figure 5.2). The substrate
binds the enzyme, forming an enzyme–substrate (ES) complex. Binding is thought
to cause a conformational change in the enzyme (induced fit model) that allows
catalysis. ES is converted to an enzyme–product (EP) complex that subsequently
dissociates to enzyme and product.
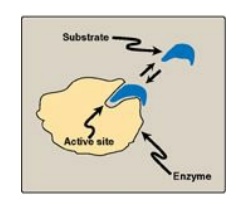
Figure 5.2 Schematic representation of an enzyme with one active site binding a substrate molecule.
B. Catalytic efficiency
Enzyme-catalyzed
reactions are highly efficient, proceeding from 103–108
times faster than uncatalyzed reactions. The number of molecules of substrate
converted to product per enzyme molecule per second is called the turnover
number, or k cat, and typically is 102–104s-1.
C. Specificity
Enzymes are highly
specific, interacting with one or a few substrates and catalyzing only one type
of chemical reaction. The set of enzymes made in a cell determines which
reactions occur in that cell.
D. Holoenzymes, apoenzymes, cofactors, and
coenzymes
Some enzymes require
molecules other than proteins for enzymic activity. The term holoenzyme refers
to the active enzyme with its nonprotein component, whereas the enzyme without
its nonprotein moiety is termed an apoenzyme and is inactive. If the nonprotein
moiety is a metal ion, such as Zn2+ or Fe2+, it is called
a cofactor. If it is a small organic molecule, it is termed a coenzyme.
Coenzymes that only transiently associate with the enzyme are called
cosubstrates. Cosubstrates dissociate from the enzyme in an altered state (NAD+
is an example). If the coenzyme is permanently associated with the enzyme and
returned to its original form, it is called a prosthetic group (FAD is an example).
Coenzymes commonly are derived from vitamins. For example, NAD+
contains niacin, and FAD contains riboflavin (see Chapter 28).
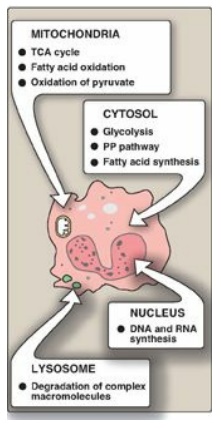
Figure 5.3 The intracellular
location of some important biochemical pathways. TCA = tricarboxylic acid; PP =
pentose phosphate.
E. Regulation
Enzyme activity can be
regulated, that is, increased or decreased, so that the rate of product
formation responds to cellular need.
F. Location within the cell
