How Enzymes Work
| Home | | Biochemistry |Chapter: Biochemistry : Enzymes
The mechanism of enzyme action can be viewed from two different perspectives. The first treats catalysis in terms of energy changes that occur during the reaction.
HOW ENZYMES WORK
The mechanism of enzyme
action can be viewed from two different perspectives. The first treats
catalysis in terms of energy changes that occur during the reaction. That is,
enzymes provide an alternate, energetically favorable reaction pathway
different from the uncatalyzed reaction. The second perspective describes how
the active site chemically facilitates catalysis.
A. Energy changes occurring during the reaction
Virtually all chemical
reactions have an energy barrier separating the reactants and the products.
This barrier, called the free energy of activation, is the energy difference
between that of the reactants and a high-energy intermediate that occurs during
the formation of product. For example, Figure 5.4 shows the changes in energy
during the conversion of a molecule of reactant A to product B as it proceeds
through the transition state (high-energy intermediate), T*:
A ↔ T*
↔ B
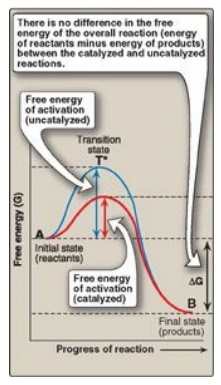
Figure 5.4 Effect of an enzyme on the activation energy of a reaction.
1. Free energy of activation: The peak of energy in Figure 5.4
is the difference in free energy between the reactant and T*, where the
high-energy intermediate is formed during the conversion of reactant to
product. Because of the high free energy of activation, the rates of
uncatalyzed chemical reactions are often slow.
2. Rate of reaction: For molecules to react, they must
contain sufficient energy to overcome the energy barrier of the transition
state. In the absence of an enzyme, only a small proportion of a population of
molecules may possess enough energy to achieve the transition state between
reactant and product. The rate of reaction is determined by the number of such
energized molecules. In general, the lower the free energy of activation, the
more molecules have sufficient energy to pass through the transition state,
and, therefore, the faster the rate of the reaction.
3. Alternate reaction pathway: An enzyme allows a reaction to proceed rapidly under conditions prevailing in the cell by providing an alternate reaction pathway with a lower free energy of activation (see Figure 5.4). The enzyme does not change the free energies of the reactants or products and, therefore, does not change the equilibrium of the reaction. It does, however, accelerate the rate by which equilibrium is reached.
B. Chemistry of the active site
The active site is not
a passive receptacle for binding the substrate but, rather, is a complex
molecular machine employing a diversity of chemical mechanisms to facilitate
the conversion of substrate to product. A number of factors are responsible for
the catalytic efficiency of enzymes, including the following examples.
1. Transition-state stabilization: The active site often acts as a
flexible molecular template that binds the substrate and initiates its
conversion to the transition state, a structure in which the bonds are not like
those in the substrate or the product (see T* at the top of the curve in Figure
5.4). By stabilizing the transition state, the enzyme greatly increases the
concentration of the reactive intermediate that can be converted to product
and, thus, accelerates the reaction. [Note: The transition state cannot be
isolated.]
2. Other mechanisms: The active site can provide
catalytic groups that enhance the probability that the transition state is
formed. In some enzymes, these groups can participate in general acid–base
catalysis in which amino acid residues provide or accept protons. In other
enzymes, catalysis may involve the transient formation of a covalent ES
complex. [Note: The mechanism of action of chymotrypsin, an enzyme of protein
digestion in the intestine, includes general base, general acid, and covalent
catalysis. A histidine at the active site of the enzyme gains (general base)
and loses (general acid) protons, mediated by the pK of histidine in proteins
being close to physiologic pH. Serine at the active site forms a covalent link
with the substrate.]
3. Visualization of the transition state: The enzyme-catalyzed conversion of
substrate to product can be visualized as being similar to removing a sweater
from an uncooperative infant (Figure 5.5). The process has a high energy of
activation because the only reasonable strategy for removing the garment (short
of ripping it off) requires that the random flailing of the baby results in
both arms being fully extended over the head, an unlikely posture. However, we
can envision a parent acting as an enzyme, first coming in contact with the
baby (forming ES), then guiding the baby’s arms into an extended, vertical
position, analogous to the ES transition state. This posture (conformation) of
the baby facilitates the removal of the sweater, forming the disrobed baby,
which here represents product. [Note: The substrate bound to the enzyme (ES) is
at a slightly lower energy than unbound substrate (S) and explains the small
“dip” in the curve at ES.]
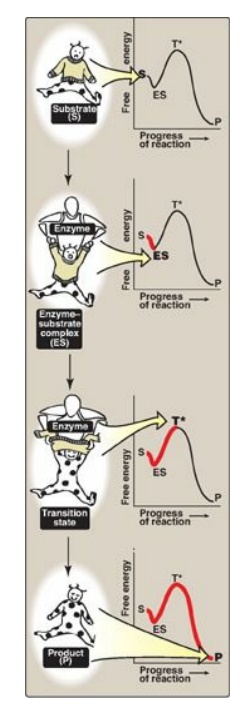
Figure 5.5 Schematic
representation of energy changes accompanying formation of an enzyme-substrate
complex and subsequent formation of a transition state.
Related Topics
