Nomenclature
| Home | | Biochemistry |Chapter: Biochemistry : Enzymes
Each enzyme is assigned two names. The first is its short, recommended name, convenient for everyday use. The second is the more complete systematic name, which is used when an enzyme must be identified without ambiguity.
NOMENCLATURE
Each enzyme is assigned
two names. The first is its short, recommended name, convenient for everyday
use. The second is the more complete systematic name, which is used when an
enzyme must be identified without ambiguity.
A. Recommended name
Most commonly used
enzyme names have the suffix “-ase” attached to the substrate of the reaction
(for example, glucosidase and urease) or to a description of the action
performed (for example, lactate dehydrogenase and adenylyl cyclase). [Note:
Some enzymes retain their original trivial names, which give no hint of the
associated enzymic reaction, for example, trypsin and pepsin.]
B. Systematic name
In the systematic
naming system, enzymes are divided into six major classes (Figure 5.1), each
with numerous subgroups. For a given enzyme, the suffix -ase is attached to a
fairly complete description of the chemical reaction catalyzed, including the
names of all the substrates, for example, lactate:NAD+
oxidoreductase. [Note: Each enzyme is also assigned a classification number.
Lactate:NAD+ oxidoreductase, for example, is 1.1.1.27.] The
systematic names are unambiguous and informative but are frequently too
cumbersome to be of general use.
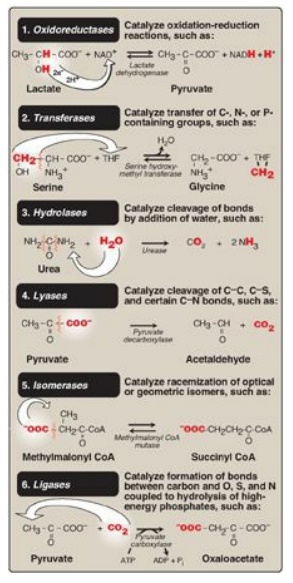
Figure 5.1 The six major
classes of enzymes with examples. NAD(H) = nicotinamide adenine dinucleotide;
THF = tetrahydrofolate; CoA = coenzyme A.
Potentially confusing enzyme nomenclature: synthetase (requires ATP), synthase (no ATP required); phosphatase (uses water to remove phosphoryl group), phosphorylase (uses Pi to break a bond and generate a phosphorylated product); dehydrogenase (NAD+/FAD is an electron acceptor in a redox reaction), oxidase (O2 is the acceptor, and oxygen atoms are not incorporated into substrate), oxygenase (one or both oxygen atoms are incorporated).
Related Topics
