Gene Therapy
| Home | | Biochemistry |Chapter: Biochemistry : Biotechnology and Human Disease
The goal of gene therapy is to treat disease through insertion of the normal, cloned DNA for a gene into the somatic cells of a patient who has a defect in that gene as a result of a disease-causing mutation.
GENE THERAPY
The goal of gene
therapy is to treat disease through insertion of the normal, cloned DNA for a
gene into the somatic cells of a patient who has a defect in that gene as a
result of a disease-causing mutation. Because somatic gene therapy changes only
the targeted somatic cells, the change is not passed on to the next generation.
[Note: In germline gene therapy, it is the germ cells that are modified, and so
the change is passed on. A long-standing moratorium on germline gene therapy is
in effect world-wide.] There are two types of gene transfer: 1) ex vivo, in
which cells from the patient are removed, transduced, and returned; and 2) in
vivo, in which the cells are directly transduced. Both types require use of a
vector (viral or nonviral) to deliver the DNA into the target cell. Challenges
of gene therapy include development of vectors, achievement of long-lived
expression, and prevention of side effects such as an immune response. The
first successful gene therapy (1990) involved two patients with severe combined
immunodeficiency disease (SCID) caused by mutations to the gene for adenosine
deaminase. It utilized mature T lymphocytes transduced ex vivo with a viral
vector (Figure 33.25). Since 1990, only a small number of patients (with a
variety of disorders, such as hemophilia, cancers, and certain types of
blindness) have been treated with gene therapy, with varying degrees of
success.
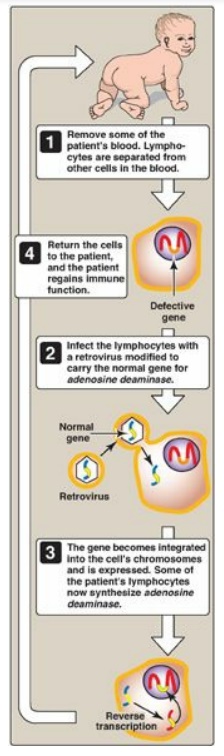
Figure 33.25 Gene therapy for
SCID caused by adenosine deaminase deficiency. [Note: Bone marrow stem cells
and a modified retroviral vector are now used.]
Related Topics
