Renal Excretion of Drugs
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Excretion of Drugs
Almost all drugs and their metabolites are excreted by the kidneys to some extent or the other. Some drugs such as gentamicin are exclusively eliminated by renal route only.
RENAL EXCRETION OF DRUGS
Almost all drugs and their metabolites are excreted
by the kidneys to some extent or the other. Some drugs such as gentamicin are
exclusively eliminated by renal route only.
Agents that are excreted in urine are –
1. Water-soluble.
2. Non-volatile.
3. Small in molecular size (less
than 500 Daltons).
4. The ones that are metabolised
slowly.
The basic functional unit of kidney involved in
excretion is the nephron. Each
kidney comprises of one million nephrons. Each nephron is made up of the
glomerulus, the proximal tubule, the loop of Henle, the distal tubule and the
collecting tubule.
The principal
processes that determine the urinary excretion of a drug are –
1. Glomerular filtration.
2. Active tubular secretion.
3. Active or passive tubular reabsorption.
These processes are depicted in Fig.6.1.
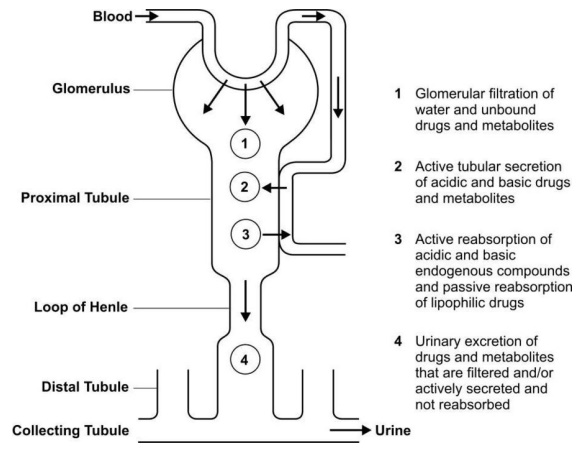
Fig. 6.1 A simplified diagram illustrating
processes involved in the urinary excretion of drugs
Glomerular filtration and active tubular secretion
tend to increase the concentration of drugs in lumen and hence facilitate
excretion whereas tubular reabsorption decreases it and prevents the movement
of drug out of the body. Thus, the rate of excretion can be given by equation:
Rate of Excretion = Rate of Filtration + Rate of
Secretion – Rate of Reabsoprtion (6.1)
Glomerular Filtration
Glomerular filtration is a non-selective,
unidirectional process whereby most compounds, ionised or unionised, are
filtered except those that are bound to plasma proteins or blood cells and thus
behave as macromolecules. The glomerulus also acts as a negatively charged
selective barrier promoting retention of anionic compounds. The driving force
for filtration through the glomerulus is the hydrostatic pressure of the blood
flowing in the capillaries. Out of the 25% of cardiac output or 1.2 litres of
blood/min that goes to the kidneys via renal artery, only 10% or 120 to 130
ml/min is filtered through the glomeruli, the rate being called as the glomerular filtration rate (GFR).
Though some 180 litres of protein and cell free ultrafiltrate pass through the
glomeruli each day, only about 1.5 litres is excreted as urine, the remainder
being reabsorbed from the tubules.
The GFR can be determined by an agent that is
excreted exclusively by filtration and is neither secreted nor reabsorbed in
the tubules. The excretion rate value of such an agent is 120 to 130 ml/min.
Creatinine, inulin, mannitol and sodium thiosulphate are used to estimate GFR
of which the former two are widely used to estimate renal function.
Active Tubular Secretion
It is a carrier-mediated process which requires
energy for transportation of compounds against the concentration gradient. The
system is capacity-limited and saturable. Two active tubular secretion
mechanisms have been identified:
1. System for secretion of organic acids/anions like penicillins, salicylates, glucuronides,
sulphates, etc. It is the same system by which endogenous acids such as uric
acid are secreted.
2. System for secretion of organic bases/cations like morphine, mecamylamine, hexamethonium
and endogenous amines such as catecholamines, choline, histamine, etc.
Both the systems are relatively non-selective and
independent of each other but both can be bidirectional i.e. agents may both be
secreted as well as reabsorbed actively, for example, uric acid.
Active secretion is unaffected by changes in pH and
protein binding since the bound drug rapidly dissociates the moment the unbound
drug gets excreted. But in contrast to glomerular filtration, it is dependent
upon renal blood flow. Drugs undergoing active secretion have excretion rate
values greater than the normal GFR value of 130 ml/min; for example, penicillin
has renal clearance value of 500 ml/min. Such a high value is indicative of
both glomerular filtration as well as tubular secretion.
Agents that are used to measure active tubular
secretion are the ones that are filtered as well as secreted to such an extent
that they are removed from the blood in a single pass through the kidneys i.e.
their clearance reflects the renal plasma flow rate which is 600 to 700 ml/min.
Para amino hippuric acid (PAH), a highly polar agent and iodopyracet are used
to determine active secretion. Active secretion occurs predominantly in the
proximal tubule region of the nephron.
Two structurally similar drugs having similar ionic
charge and employing the same carrier-mediated process for excretion enter into
competition. A drug with greater rate
of clearance will retard the excretion of the other drug with which it
competes. The half-life of both the drugs is increased since the total sites
for active secretion are limited. This may result in accumulation of drugs and
thus, precipitation of toxicity. However, the principle of competition can be
exploited for therapeutic benefits. An interesting example of this is the
anionic agent probenecid. Probenecid
inhibits the active tubular secretion of organic acids such as penicillins,
PAS, PAH, 17-keto steroids, etc. thus increasing their concentration in plasma
by at least two fold. A 50% reduction in penicillin G dose is suggested,
especially when the drug is meant to be consumed in large doses as in
gonococcal infections. The actively secreted and filtered probenecid, if
unionised in tubular fluid, is highly lipid soluble and therefore will get
reabsorbed passively. Inhibition of drug secretion by probenecid is undesirable
in case of nitrofurantoin since the latter is used as a urinary tract
antiseptic (organic bases can also interfere with tubular secretion of cationic
drugs but are not in therapeutic use). While inhibiting the active secretion of
anionic drugs on one hand, probenecid is known to suppress the carrier-mediated
reabsorption of the endogenous metabolite, uric acid and is thus of therapeutic
value as a uricosuric agent in the treatment of gout. Just as probenicid is a
competitive inhibitor of organic anion transport, cimetidine is a competitive
inhibitor of organic cation transport.
Tubular Reabsorption
Tubular reabsorption occurs after the glomerular
filtration of drugs. It takes place all along the renal tubule. Reabsorption of
a drug is indicated when the excretion rate values are less than the GFR of 130
ml/min. An agent such as glucose that is completely reabsorbed after filtration
has a clearance value of zero. Contrary
to tubular secretion, reabsorption results in an increase in the half-life of a drug.
Tubular reabsorption can either be an:
1. Active process, or
2. Passive process.
Active tubular reabsorption is
commonly seen with high threshold endogenous substances or nutrients that the body needs to conserve such as
electrolytes, glucose, vitamins, amino acids, etc. Uric acid is also actively
reabsorbed (inhibited by the uricosuric agents). Very few drugs are known to
undergo reabsorption actively e.g. oxopurinol.
Passive tubular reabsorption is common
for a large number of exogenous substances
including drugs. The driving force for such a process i.e. the
concentration gradient is established by the back diffusion or reabsorption of
water along with sodium and other inorganic ions. Understandably, if a drug is
neither secreted nor reabsorbed, its concentration in the urine will be 100
times that of free drug in plasma due to water reabsorption since less than 1%
of glomerular filtrate is excreted as urine.
The primary determinant in the passive reabsorption
of drugs is their lipophilicity. Lipophilic substances are extensively
reabsorbed while polar molecules are not. Since a majority of drugs are weak
electrolytes (weak acids or weak bases), diffusion of such agents through the
lipoidal tubular membrane depend upon the degree of ionisation which in turn
depends on three factors:
1. pH of the urine.
2. pKa of the drug.
3. Urine flow rate.
Urine pH: It is an important factor in the
sense that it is not constant like the plasma pH but varies between 4.5 to 7.5, the two extremes. Thus, a large pH
gradient may exist between urine and plasma.
The pH of the urine is dependent upon diet, drug
intake and pathophysiology of the patient. Food rich in carbohydrates result in
higher urinary pH whereas proteins lower it. Drugs such as acetazolamide and
antacids such as sodium bicarbonate produce alkaline urine while ascorbic acid
makes it acidic. More significant alteration in urine pH is brought about by
i.v. infusion of solutions of sodium bicarbonate and ammonium chloride which
are used in the treatment of acid-base imbalance. Respiratory and metabolic
acidosis and alkalosis result in acidification and alkalinisation of the urine
respectively.
The relative amount of ionised and unionised drug
in the urine at a particular pH and the percent of drug ionised at this pH can
be computed from the Henderson-Hasselbach
equations:
for weak acids,
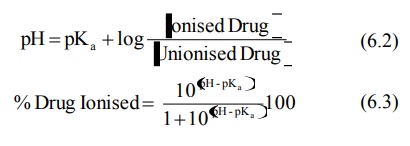
for weak bases,
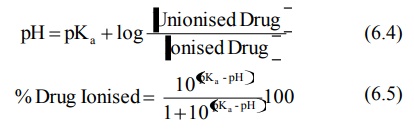
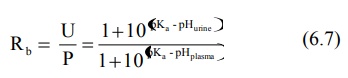
The concentration ratio R of the drug in urine to
that in plasma (U : P) can be given by equations derived by Shore et al:
for weak acids,
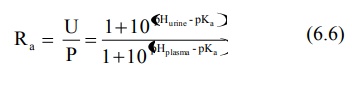
for weak bases,
Note : The above equations from
6.2 to 6.7 are identical to equations 2.10 to 2.15 of chapter 2.
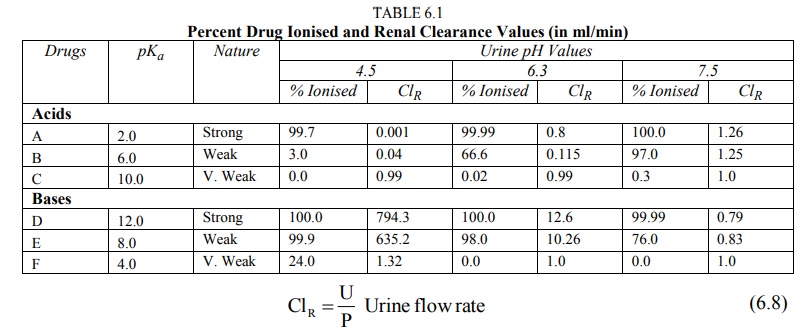
The relationship between drug pKa, urine
pH, degree of ionisation and renal clearance is illustrated in Table 6.1. Table
6.1 shows percent drug ionised and renal clearance values (in ml/min) of
several acidic and basic drugs at various values of urine pH, assuming that the
drug does not bind to plasma proteins, urine flow of 1 ml/min, plasma pH 7.4
and that equilibrium is achieved by diffusion of unionised drug only. The renal
clearance values ClR are computed by use of equation 6.8.
Drug pKa: The
significance of pH dependent excretion for any particular compound is greatly dependent upon its pKa
and lipid solubility. A characteristic of drugs, pKa values govern
the degree of ionisation at a particular pH. A polar and ionised drug will be
poorly reabsorbed passively and excreted rapidly (see Table 6.1). Reabsorption is also affected by the lipid
solubility of drug; an ionised but lipophilic drug will be reabsorbed while an
unionised but polar one will be excreted.
The combined effect of urine pH and drug pKa
and lipid solubility on reabsorption of drugs is summarized as follows:
1. An acidic drug such as
penicillin or a basic drug such as gentamicin which is polar in its unionised
form, is not reabsorbed passively, irrespective of the extent of ionisation in
urine. Excretion of such drugs is independent of pH of urine and its flow rate.
Their rate of excretion is the sum of rate of filtration and rate of active
secretion.
2. Very weakly acidic, nonpolar
drugs (pKa > 8.0) such as phenytoin or very weakly basic,
nonpolar drugs (pKa < 6.0) such as propoxyphene are mostly
unionised throughout the entire range of urine pH and are therefore extensively
reabsorbed passively at all values of urine pH. The rate of excretion of such
drugs is always low and insensitive to urine pH.
3. A strongly acidic drug (pKa
< 2.0) such as cromoglycic acid or a strongly basic drug (pKa
> 12.0) such as guanethidine, is completely ionised at all values of
urine pH and are, therefore, not reabsorbed. Their rate of excretion is always
high and insensitive to pH of urine.
4. Only for an acidic drug in the
pKa range 3.0 to 8.0 (e.g. several NSAIDs) and for a basic drug in
the pKa range 6.0 to 12.0 (e.g. morphine analogs, tricyclic antidepressants,
etc.) the extent of reabsorption is greatly dependent upon urine pH and varies
from negligible to almost complete; for example, the amount of dexamphetamine
excreted in the urine varies from 3 to 55% of the administered dose as the
urine pH varies from 8.0 to 5.0. Fig. 6.2 illustrates the influence of urine pH
on drug excretion.
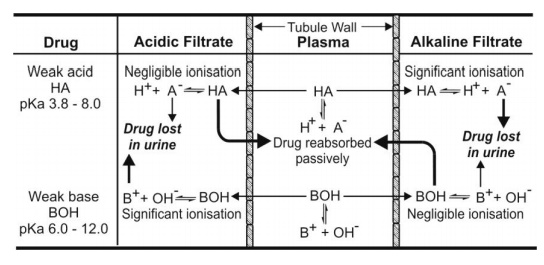
Fig. 6.2. Influence of urinary pH on
excretion of weakly acidic and weakly basic drugs. Bold arrows indicate that the process is
predominant.
The toxicity due to overdosage of drugs whose
excretion is sensitive to pH change can be treated by acidification or
alkalinisation of the urine with ammonium chloride or sodium bicarbonate
respectively. Thus, crystalluria caused by precipitation of sulphonamides in
the renal tubules and subsequent kidney damage can be overcome by alkalinising
the urine. Excretion of basic drugs can be promoted by acidification of urine.
The therapeutic activity of the urinary antiseptic hexamine also depends on the
urine pH. It is not converted to active form i.e. formaldehyde unless the urine
is acidic.
Urine Flow Rate: In addition to urine pH and drug
pKa, the rate of urine flow also
influences the extent of reabsorption. Polar drugs whose excretion is
independent of urine pH and are not reabsorbed, are unaffected by urine flow
rate. An increase in urine flow in case of such drugs will only produce more
dilute urine. Only those drugs whose reabsorption is pH-sensitive, for example,
weak acids and weak bases, show dependence on urine flow rate. For such agents,
reabsorption is inversely proportional to the urinary flow. These compounds can
be divided into two types based on their extent of reabsorption in relation to
that of water:
1. Drugs which are reabsorbed to
an extent equal to or greater than the reabsorption of water e.g.
phenobarbital. In such cases, the relationship between renal clearance and
urinary excretion is linear.
2. Drugs which are reabsorbed to
an extent lower than the reabsorption of water e.g. theophylline and many more
drugs. In these cases, the relationship between renal clearance and urinary
excretion is convex curvilinear.
Urine flow rate can be increased by forced
diuresis. Forced diuresis is the increase in urine flow induced by large fluid intake or administration of mannitol
or other diuretics. The principle can be used in an intoxicated person to
remove excessive drug by promoting its excretion and decreasing the time for
reabsorption.
Both urine pH control and forced diuresis can be
used to treat toxicity with drug overdose when –
1. Urinary excretion is the major
route for elimination of drug.
2. The drug is extensively
reabsorbed passively from the renal tubules.
3. The reabsorption is sensitive
to urine pH (and urine flow rate).
Apart from the foregoing discussion on the passive
reabsorption of drugs, the process is also important in the reabsorption of low
threshold substances such as urea, certain phosphates and sulphates, etc.
Related Topics
