Chemical factors of Drug
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Biotransformation of Drugs
Chemical factors of Drug: Induction of Drug Metabolising Enzymes, Inhibition of Drug Metabolising Enzymes, Environmental Chemicals
CHEMICAL FACTORS
Induction of Drug Metabolising Enzymes
The phenomenon of increased drug metabolising ability of the enzymes
(especially of microsomal monooxygenase system) by several drugs and chemicals
is called as enzyme induction and the agents which bring about such an effect are known as enzyme inducers.
Most enzyme inducers have following properties –
1. They are lipophilic compounds.
2. They are substrate for the
inducted enzyme system.
3. They have long elimination
half-lives.
Mechanisms involved in enzyme induction are –
1. Increase in both liver size
and liver blood flow.
2. Increase in both total and
microsomal protein content.
3. Increased stability of
enzymes.
4. Increased synthesis of
cytochrome P-450.
5. Decreased degradation of
cytochrome P-450.
6. Proliferation of smooth
endoplasmic reticulum.
Two categories of inducers have been defined –
1. Phenobarbital type inducers: includes
several drugs and pesticides which increase
the rate of metabolism of a large number of drugs. The most thoroughly
studied enzyme inducer is phenobarbital which can increase enzyme activity up
to 4 times.
2. Polycyclic hydrocarbon type inducers: such as 3-methyl cholanthrene and cigarette smoke which stimulate the metabolic rate of few drugs.
Some drugs such as carbamazepine, meprobamate, cyclophosphamide,
rifampicin, etc. stimulate their own metabolism, the phenomenon being called as
auto-induction or self-induction.
The most thoroughly studied enzyme inducer is
phenobarbital which can increase enzyme activity up to 4 times. An example
which shows that enzyme induction can have serious consequences in clinical
practice is the inducing effect of phenobarbital on dicoumarol levels. Extreme
caution must be exercised when phenobarbital and dicoumarol are co-administered
to avoid either failure of the anticoagulant therapy or haemorrhagic crises.
Consequences of enzyme induction includes –
·
Decrease in pharmacological
activity of drugs
·
Increased activity where the
metabolites are active, and
·
Altered physiologic status due to
enhanced metabolism of endogenous compounds such as sex hormones.
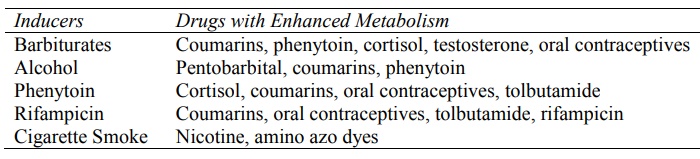
Some examples of inducers and drugs affected by
them are given in Table 5.4.
TABLE 5.4
Inducers of Drug Metabolising Enzyme System and Drugs Commonly Affected
by Them
Inhibition of Drug Metabolising Enzymes
A decrease in the drug metabolising ability of an enzyme is called as enzyme inhibition.
The process of inhibition may be direct or
indirect.
1. Direct Inhibition: may
result from interaction at the enzymic site, the net outcome being a change in enzyme activity.
Direct enzyme inhibition can occur by one of the 3
mechanisms –
a. Competitive Inhibition: results when structurally similar compounds compete for the same
site on an enzyme. Such an inhibition due to substrate competition is reversible and can be overcome by high
concentration of one of the substrates, e.g. methacholine inhibits metabolism
of acetylcholine by competing with it for cholinesterase.
b. Non-competitive Inhibition: results when a structurally unrelated agent interacts with the
enzyme and prevents the metabolism of drugs. Since the interaction is not
structure-specific, metals like lead, mercury and arsenic and organophosphorus
insecticides inhibit the enzymes non-competitively. Isoniazid inhibits the
metabolism of phenytoin by the same mechanism.
c. Product Inhibition: results when the metabolic product competes with
the substrate for the same enzyme. The phenomenon is also called as autoinhibition.
Certain specific inhibitors such as xanthine
oxidase inhibitors (e.g. allopurinol) and MAO inhibitors (e.g. phenelzine) also
inhibit the enzyme activity directly. Direct enzyme inhibition is usually
rapid; a single dose of inhibitor may be sufficient to demonstrate enzyme
inhibition.
2. Indirect Inhibition: is
brought about by one of the two mechanisms
–
a. Repression: is defined as the decrease in enzyme content. It may be due to a fall
in
the rate of enzyme synthesis as affected by ethionine, puromycin and
actinomycin D or because of rise in the rate of enzyme degradation such as by
carbon tetrachloride, carbon disulphide, disulphiram, etc.
b. Altered Physiology: due to nutritional deficiency or hormonal imbalance.
Enzyme inhibition is more
important clinically than enzyme induction, especially for drugs with narrow therapeutic
index, e.g. anticoagulants, antiepileptics, hypoglycaemics, since it results in
prolonged pharmacological action with increased possibility of precipitation of
toxic effects.
Some examples of inhibitors and drugs affected by
them are given in Table 5.5.
TABLE 5.5
Enzyme Inhibitors and Drugs Affected by them

Inhibitors : Drugs with Decreased Metabolism
MAO inhibitors :
Barbiturates, tyramine
Coumarins : Phenytoin
Allopurinol :
6-Mercaptopurine
PAS : Phenytoin,
hexobarbital
An important example of clinically significant
enzyme inhibition is the effect of phenylbutazone on warfarin plasma levels.
Warfarin is used as racemic mixture, with the S isomer being 5 times more active than the R isomer. Moreover, warfarin is eliminated from the body almost
exclusively by metabolism and the S
isomer is metabolised more rapidly that the R
isomer. The effect of phenylbutazone on increased levels of warfarin can thus
be concluded as –
·
Increase in R isomer levels is due to its displacement from the plasma proteins
(little increase in anticoagulant activity).
·
Increase in S isomer levels is due to inhibition of metabolism, with tremendous
increase in anticoagulant activity and tendency to cause haemorrhage.
Environmental Chemicals
Several environmental agents influence the drug
metabolising ability of enzymes.
·
Halogenated pesticides such as
DDT and polycyclic aromatic hydrocarbons contained in cigarette smoke have
enzyme induction effect.
·
Organophosphate insecticides and
heavy metals such as mercury, tin, nickel, cobalt and arsenic inhibit drug
metabolising ability of enzymes.
Other environmental factors that may influence drug
metabolism are temperature, altitude, pressure, atmosphere, etc.
Related Topics
