Secondary Structure of Proteins
| Home | | Biochemistry |Chapter: Biochemistry : Structure of Proteins
The polypeptide backbone does not assume a random three-dimensional structure but, instead, generally forms regular arrangements of amino acids that are located near each other in the linear sequence.
SECONDARY STRUCTURE OF PROTEINS
The polypeptide
backbone does not assume a random three-dimensional structure but, instead,
generally forms regular arrangements of amino acids that are located near each
other in the linear sequence. These arrangements are termed the secondary
structure of the polypeptide. The α-helix, β-sheet, and β-bend (β-turn) are
examples of secondary structures commonly encountered in proteins. [Note: The
collagen α-chain helix, another example of secondary structure.
A. α-Helix
Several different
polypeptide helices are found in nature, but the α-helix is the most common. It
is a spiral structure, consisting of a tightly packed, coiled polypeptide
backbone core, with the side chains of the component amino acids extending
outward from the central axis to avoid interfering sterically with each other
(Figure 2.6). A very diverse group of proteins contains α-helices. For example,
the keratins are a family of closely related, fibrous proteins whose structure
is nearly entirely α-helical. They are a major component of tissues such as
hair and skin, and their rigidity is determined by the number of disulfide
bonds between the constituent polypeptide chains. In contrast to keratin,
myoglobin, whose structure is also highly α-helical, is a globular, flexible
molecule.
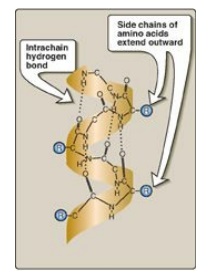
Figure 2.6
α-Helix showing peptide backbone.
1. Hydrogen bonds: An α-helix is stabilized by
extensive hydrogen bonding between the peptide-bond carbonyl oxygens and amide
hydrogens that are part of the polypeptide backbone (see Figure 2.6). The
hydrogen bonds extend up and are parallel to the spiral from the carbonyl
oxygen of one peptide bond to the –I
NH I– group of a peptide linkage
four residues ahead in the polypeptide. This insures that all but the first and
last peptide bond components are linked to each other through intrachain
hydrogen bonds. Hydrogen bonds are individually weak, but they collectively
serve to stabilize the helix.
2. Amino acids per turn: Each turn of an α-helix contains
3.6 amino acids. Thus, amino acid residues spaced three or four residues apart
in the primary sequence are spatially close together when folded in the
α-helix.
3. Amino acids that disrupt an α-helix: Proline disrupts an α-helix because its secondary amino group is not geometrically compatible with the right-handed spiral of the α-helix. Instead, it inserts a kink in the chain, which interferes with the smooth, helical structure. Large numbers of charged amino acids (for example, glutamate, aspartate, histidine, lysine, and arginine) also disrupt the helix by forming ionic bonds or by electrostatically repelling each other. Finally, amino acids with bulky side chains, such as tryptophan, or amino acids, such as valine or isoleucine, that branch at the β-carbon (the first carbon in the R group, next to the α-carbon) can interfere with formation of the α-helix if they are present in large numbers.
B. β-Sheet
The β-sheet is another form of secondary structure in which all of the peptide bond components are involved in hydrogen bonding (Figure 2.7A). The surfaces of β-sheets appear “pleated,” and these structures are, therefore, often called β-pleated sheets. When illustrations are made of protein structure, β-strands are often visualized as broad arrows (Figure 2.7B).
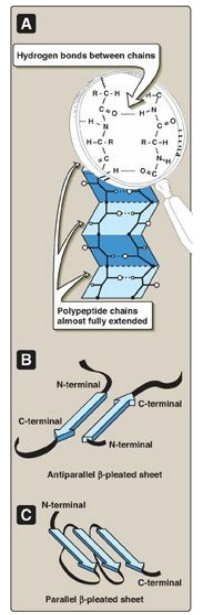
Figure 2.7 A. Structure of a β-sheet. B. An antiparallel β-sheet with the β-strands represented as broad arrows. C. A parallel β-sheet formed from a single polypeptide chain folding back on itself.
1. Comparison of a β-sheet and an α-helix: Unlike the α-helix, β-sheets are
composed of two or more peptide chains (β-strands), or segments of polypeptide
chains, which are almost fully extended. Note also that the hydrogen bonds are
perpendicular to the polypeptide backbone in β-sheets (see Figure 2.7A).
2. Parallel and antiparallel sheets: A β-sheet can be formed from two
or more separate polypeptide chains or segments of polypeptide chains that are
arranged either antiparallel to each other (with the N-terminal and C-terminal
ends of the β-strands alternating as shown in Figure 2.7B) or parallel to each
other (with all the N-termini of the β-strands together as shown in Figure
2.7C). When the hydrogen bonds are formed between the polypeptide backbones of
separate polypeptide chains, they are termed interchain bonds. A β-sheet can
also be formed by a single polypeptide chain folding back on itself (see Figure
2.7C). In this case, the hydrogen bonds are intrachain bonds. In globular
proteins, β-sheets always have a right-handed curl, or twist, when viewed along
the polypeptide backbone. [Note: Twisted β-sheets often form the core of
globular proteins.]
The α-helix and β-sheet structures provide maximal
hydrogen bonding for peptide bond components within the interior of
polypeptides.
C. β-Bends (reverse turns, β-turns)
β-Bends reverse the
direction of a polypeptide chain, helping it form a compact, globular shape.
They are usually found on the surface of protein molecules and often include
charged residues. [Note: β-Bends were given this name because they often
connect successive strands of antiparallel β-sheets.] β-Bends are generally
composed of four amino acids, one of which may be proline, the amino acid that
causes a kink in the polypeptide chain. Glycine, the amino acid with the
smallest R group, is also frequently found in β-bends. β-Bends are stabilized
by the formation of hydrogen and ionic bonds.
D. Nonrepetitive secondary structure
Approximately one half
of an average globular protein is organized into repetitive structures, such as
the α-helix and β-sheet. The remainder of the polypeptide chain is described as
having a loop or coil conformation. These nonrepetitive secondary structures
are not random, but rather simply have a less regular structure than those
described above. [Note: The term “random coil” refers to the disordered
structure obtained when proteins are denatured.]
E. Supersecondary structures (motifs)
Globular proteins are
constructed by combining secondary structural elements (that is, α-helices,
β-sheets, and coils), producing specific geometric patterns or motifs. These
form primarily the core (interior) region of the molecule. They are connected by
loop regions (for example, β-bends) at the surface of the protein.
Supersecondary structures are usually produced by the close packing of side
chains from adjacent secondary structural elements. Thus, for example,
α-helices and β-sheets that are adjacent in the amino acid sequence are also
usually (but not always) adjacent in the final, folded protein. Some of the
more common motifs are illustrated in Figure 2.8.
Motifs may be associated with particular functions.
Proteins that bind to DNA contain a limited number of motifs. The
helix-loop-helix motif is an example found in a number of proteins that
function as transcription factors.
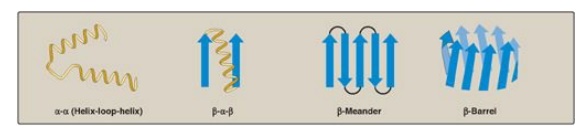
Figure 2.8 Some common structural motifs involving β-helices and β-sheets. The names describe their schematic appearance.
Related Topics
