Tertiary Structure of Globular Proteins
| Home | | Biochemistry |Chapter: Biochemistry : Structure of Proteins
The primary structure of a polypeptide chain determines its tertiary structure. “Tertiary” refers both to the folding of domains (the basic units of structure and function, see discussion below), and to the final arrangement of domains in the polypeptide.
The primary structure
of a polypeptide chain determines its tertiary structure. “Tertiary” refers
both to the folding of domains (the basic units of structure and function, see
discussion below), and to the final arrangement of domains in the polypeptide.
The structure of globular proteins in aqueous solution is compact, with a high
density (close packing) of the atoms in the core of the molecule. Hydrophobic
side chains are buried in the interior, whereas hydrophilic groups are
generally found on the surface of the molecule.
A. Domains
Domains are the fundamental functional and three-dimensional structural units of polypeptides. Polypeptide chains that are greater than 200 amino acids in length generally consist of two or more domains. The core of a domain is built from combinations of supersecondary structural elements (motifs). Folding of the peptide chain within a domain usually occurs independently of folding in other domains. Therefore, each domain has the characteristics of a small, compact globular protein that is structurally independent of the other domains in the polypeptide chain.
B. Interactions stabilizing tertiary structure
The unique
three-dimensional structure of each polypeptide is determined by its amino acid
sequence. Interactions between the amino acid side chains guide the folding of
the polypeptide to form a compact structure. The following four types of
interactions cooperate in stabilizing the tertiary structures of globular
proteins.
1. Disulfide bonds: A disulfide bond is a covalent linkage formed from the sulfhydryl group (–SH) of each of two cysteine residues to produce a cystine residue (Figure 2.9). The two cysteines may be separated from each other by many amino acids in the primary sequence of a polypeptide or may even be located on two different polypeptide chains. The folding of the polypeptide chain(s) brings the cysteine residues into proximity and permits covalent bonding of their side chains. A disulfide bond contributes to the stability of the three-dimensional shape of the protein molecule and prevents it from becoming denatured in the extracellular environment. For example, many disulfide bonds are found in proteins such as immunoglobulins that are secreted by cells.
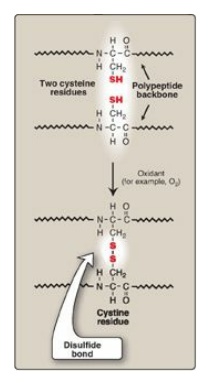
Figure 2.9 Formation of a disulfide bond by the oxidation of two cysteine residues, producing one cystine residue.
2. Hydrophobic interactions: Amino acids with nonpolar side
chains tend to be located in the interior of the polypeptide molecule, where
they associate with other hydrophobic amino acids (Figure 2.10). In contrast,
amino acids with polar or charged side chains tend to be located on the surface
of the molecule in contact with the polar solvent. [Note: Recall that proteins
located in nonpolar (lipid) environments, such as a membrane, exhibit the
reverse arrangement (see Figure 1.4).] In each case, a segregation of R groups
occurs that is energetically most favorable.
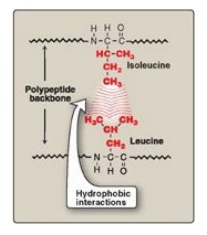
Figure 2.10 Hydrophobic interactions between amino acids with nonpolar side chains.
3. Hydrogen bonds: Amino acid side chains containing
oxygen- or nitrogen-bound hydrogen, such as in the alcohol groups of serine and
threonine, can form hydrogen bonds with electron-rich atoms, such as the oxygen
of a carboxyl group or carbonyl group of a peptide bond (Figure 2.11; see also
Figure 1.6). Formation of hydrogen bonds between polar groups on the surface of
proteins and the aqueous solvent enhances the solubility of the protein.
4. Ionic interactions: Negatively charged groups, such as
the carboxylate group (–I COO I–) in the side chain of aspartate or
glutamate, can interact with positively charged groups such as the amino group
(–I NH3+) in
the side chain of lysine (see Figure 2.11).
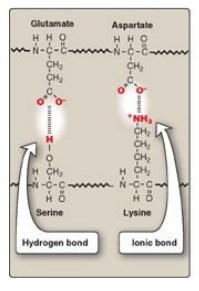
Figure 2.11 Interactions
of side chains of amino acids through hydrogen bonds and ionic bonds (salt bridges).
C. Protein folding
Interactions between
the side chains of amino acids determine how a long polypeptide chain folds
into the intricate three-dimensional shape of the functional protein. Protein
folding, which occurs within the cell in seconds to minutes, involves nonrandom,
ordered pathways. As a peptide folds, secondary structures form driven by the
hydrophobic effect (that is, hydrophobic groups come together as water is
released). These small structures combine to form larger structures. Additional
events stabilize secondary structure and initiate formation of tertiary
structure. In the last stage, the peptide achieves its fully folded, native
(functional) form characterized by a low-energy state (Figure 2.12). [Note:
Some biologically active proteins or segments thereof lack a stable tertiary
structure. They are referred to as “intrinsically disordered” proteins.]
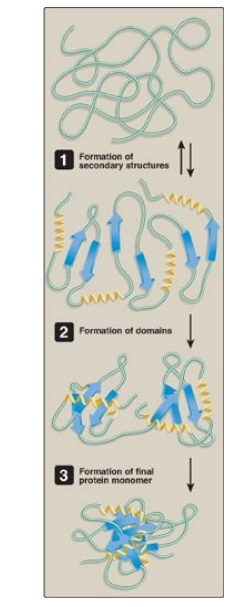
Figure 2.12 Steps in protein folding (simplified).
D. Denaturation of proteins
Protein denaturation
results in the unfolding and disorganization of a protein’s secondary and
tertiary structures without the hydrolysis of peptide bonds. Denaturing agents
include heat, organic solvents, strong acids or bases, detergents, and ions of
heavy metals such as lead. Denaturation may, under ideal conditions, be
reversible, such that the protein refolds into its original native structure
when the denaturing agent is removed. However, most proteins, once denatured,
remain permanently disordered. Denatured proteins are often insoluble and
precipitate from solution.
E. Role of chaperones in protein folding
The information needed for correct protein folding is contained in the primary structure of the polypeptide. However, most proteins when denatured do not resume their native conformations even under favorable environmental conditions. This is because, for many proteins, folding is a facilitated process that requires a specialized group of proteins, referred to as “molecular chaperones,” and adenosine triphosphate hydrolysis. The chaperones, also known as “heat shock proteins” (Hsp), interact with a polypeptide at various stages during the folding process. Some chaperones bind hydrophobic regions of an extended polypeptide and are important in keeping the protein unfolded until its synthesis is completed (for example, Hsp70). Others form cage-like macromolecular structures composed of two stacked rings. The partially folded protein enters the cage, binds the central cavity through hydrophobic interactions, folds, and is released (for example, mitochondrial Hsp60). [Note: Cage-like chaperones are sometimes referred to as “chaperonins.”] Chaperones, then, facilitate correct protein folding by binding to and stabilizing exposed, aggregation-prone hydrophobic regions in nascent (and denatured) polypeptides, preventing premature folding.
Related Topics
